We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
3lbx
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
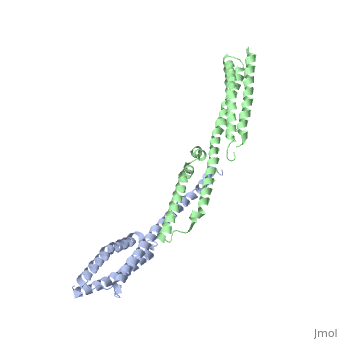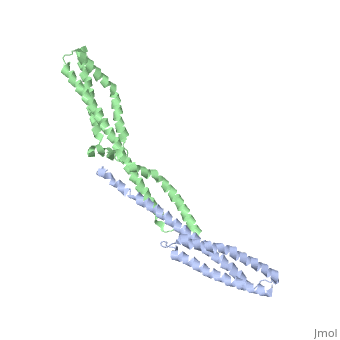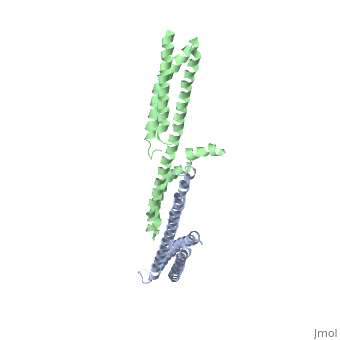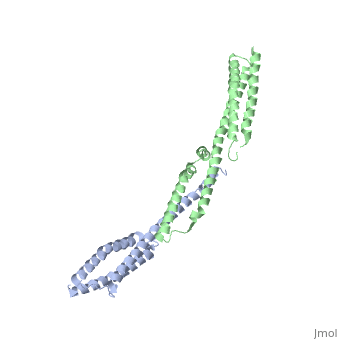
| - | + | ==Crystal Structure of the Erythrocyte Spectrin Tetramerization Domain Complex== | |
| - | === | + | <StructureSection load='3lbx' size='340' side='right' caption='[[3lbx]], [[Resolution|resolution]] 2.80Å' scene=''> |
| - | + | == Structural highlights == | |
| + | <table><tr><td colspan='2'>[[3lbx]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3LBX OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3LBX FirstGlance]. <br> | ||
| + | </td></tr><tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1owa|1owa]], [[3f31|3f31]], [[2spc|2spc]], [[1aj3|1aj3]], [[3edv|3edv]], [[3kbt|3kbt]]</td></tr> | ||
| + | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">SPTA, SPTA1 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens]), SPTB, SPTB1 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens])</td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3lbx FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3lbx OCA], [http://www.rcsb.org/pdb/explore.do?structureId=3lbx RCSB], [http://www.ebi.ac.uk/pdbsum/3lbx PDBsum]</span></td></tr> | ||
| + | </table> | ||
| + | == Disease == | ||
| + | [[http://www.uniprot.org/uniprot/SPTA1_HUMAN SPTA1_HUMAN]] Defects in SPTA1 are the cause of elliptocytosis type 2 (EL2) [MIM:[http://omim.org/entry/130600 130600]]. EL2 is a Rhesus-unlinked form of hereditary elliptocytosis, a genetically heterogeneous, autosomal dominant hematologic disorder. It is characterized by variable hemolytic anemia and elliptical or oval red cell shape.<ref>PMID:2794061</ref> <ref>PMID:8018926</ref> <ref>PMID:1679439</ref> <ref>PMID:1878597</ref> <ref>PMID:2568862</ref> <ref>PMID:1541680</ref> <ref>PMID:8364215</ref> <ref>PMID:2384601</ref> <ref>PMID:1638030</ref> <ref>PMID:2568861</ref> <ref>PMID:8193371</ref> <ref>PMID:7772539</ref> Defects in SPTA1 are a cause of hereditary pyropoikilocytosis (HPP) [MIM:[http://omim.org/entry/266140 266140]]. HPP is an autosomal recessive disorder characterized by hemolytic anemia, microspherocytosis, poikilocytosis, and an unusual thermal sensitivity of red cells.<ref>PMID:1878597</ref> Defects in SPTA1 are the cause of spherocytosis type 3 (SPH3) [MIM:[http://omim.org/entry/270970 270970]]; also known as hereditary spherocytosis type 3 (HS3). Spherocytosis is a hematologic disorder leading to chronic hemolytic anemia and characterized by numerous abnormally shaped erythrocytes which are generally spheroidal. SPH3 is characterized by severe hemolytic anemia. Inheritance is autosomal recessive. [[http://www.uniprot.org/uniprot/SPTB1_HUMAN SPTB1_HUMAN]] Defects in SPTB are the cause of elliptocytosis type 3 (EL3) [MIM:[http://omim.org/entry/182870 182870]]. EL3 is a Rhesus-unlinked form of hereditary elliptocytosis, a genetically heterogeneous, autosomal dominant hematologic disorder. It is characterized by variable hemolytic anemia and elliptical or oval red cell shape.<ref>PMID:8226774</ref> <ref>PMID:7883966</ref> <ref>PMID:8018926</ref> <ref>PMID:1975598</ref> Defects in SPTB are the cause of spherocytosis type 2 (SPH2) [MIM:[http://omim.org/entry/182870 182870]]; also known as hereditary spherocytosis type 2 (HS2). Spherocytosis is a hematologic disorder leading to chronic hemolytic anemia and characterized by numerous abnormally shaped erythrocytes which are generally spheroidal. SPH2 is characterized by severe hemolytic anemia. Inheritance is autosomal dominant. | ||
| + | == Function == | ||
| + | [[http://www.uniprot.org/uniprot/SPTA1_HUMAN SPTA1_HUMAN]] Spectrin is the major constituent of the cytoskeletal network underlying the erythrocyte plasma membrane. It associates with band 4.1 and actin to form the cytoskeletal superstructure of the erythrocyte plasma membrane. [[http://www.uniprot.org/uniprot/SPTB1_HUMAN SPTB1_HUMAN]] Spectrin is the major constituent of the cytoskeletal network underlying the erythrocyte plasma membrane. It associates with band 4.1 and actin to form the cytoskeletal superstructure of the erythrocyte plasma membrane. | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/lb/3lbx_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | As the principal component of the membrane skeleton, spectrin confers integrity and flexibility to red cell membranes. Although this network involves many interactions, the most common hemolytic anemia mutations that disrupt erythrocyte morphology affect the spectrin tetramerization domains. Although much is known clinically about the resulting conditions (hereditary elliptocytosis and pyropoikilocytosis), the detailed structural basis for spectrin tetramerization and its disruption by hereditary anemia mutations remains elusive. Thus, to provide further insights into spectrin assembly and tetramer site mutations, a crystal structure of the spectrin tetramerization domain complex has been determined. Architecturally, this complex shows striking resemblance to multirepeat spectrin fragments, with the interacting tetramer site region forming a central, composite repeat. This structure identifies conformational changes in alpha-spectrin that occur upon binding to beta-spectrin, and it reports the first structure of the beta-spectrin tetramerization domain. Analysis of the interaction surfaces indicates an extensive interface dominated by hydrophobic contacts and supplemented by electrostatic complementarity. Analysis of evolutionarily conserved residues suggests additional surfaces that may form important interactions. Finally, mapping of hereditary anemia-related mutations onto the structure demonstrate that most, but not all, local hereditary anemia mutations map to the interacting domains. The potential molecular effects of these mutations are described. | ||
| - | + | Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex.,Ipsaro JJ, Harper SL, Messick TE, Marmorstein R, Mondragon A, Speicher DW Blood. 2010 Jun 10;115(23):4843-52. Epub 2010 Mar 2. PMID:20197550<ref>PMID:20197550</ref> | |
| - | + | ||
| - | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | + | </div> | |
| - | + | ||
| - | + | ||
| - | + | ||
==See Also== | ==See Also== | ||
*[[Spectrin|Spectrin]] | *[[Spectrin|Spectrin]] | ||
| - | + | == References == | |
| - | == | + | <references/> |
| - | + | __TOC__ | |
| + | </StructureSection> | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
| - | [[Category: Harper, S L | + | [[Category: Harper, S L]] |
| - | [[Category: Ipsaro, J J | + | [[Category: Ipsaro, J J]] |
| - | [[Category: Marmorstein, R | + | [[Category: Marmorstein, R]] |
| - | [[Category: Messick, T E | + | [[Category: Messick, T E]] |
| - | [[Category: Mondragon, A | + | [[Category: Mondragon, A]] |
| - | [[Category: Speicher, D W | + | [[Category: Speicher, D W]] |
[[Category: Actin capping]] | [[Category: Actin capping]] | ||
[[Category: Actin-binding]] | [[Category: Actin-binding]] | ||
Revision as of 17:28, 18 December 2014
Crystal Structure of the Erythrocyte Spectrin Tetramerization Domain Complex
| |||||||||||
Categories: Homo sapiens | Harper, S L | Ipsaro, J J | Marmorstein, R | Messick, T E | Mondragon, A | Speicher, D W | Actin capping | Actin-binding | Alpha helix | Cell shape | Complex | Cytoskeleton | Disease mutation | Elliptocytosis | Helical linker | Hereditary hemolytic anemia | Partial repeat | Phosphoprotein | Pyropoikilocytosis | Sh3 domain | Spectrin | Structural protein | Tetramer | Three-helix bundle