We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
4ald
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
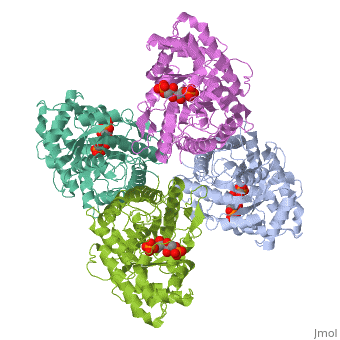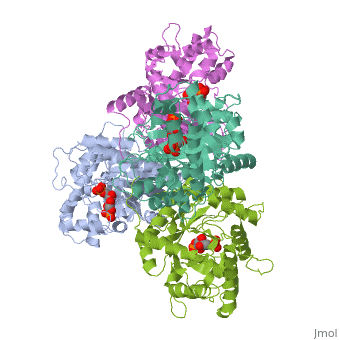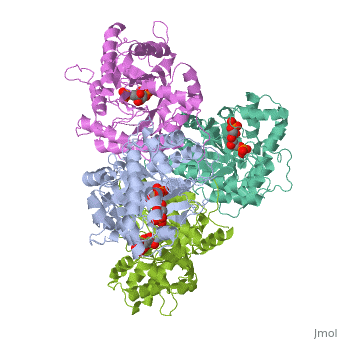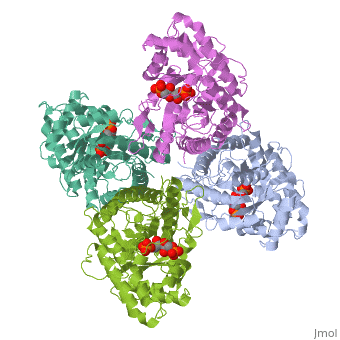
| - | + | ==HUMAN MUSCLE FRUCTOSE 1,6-BISPHOSPHATE ALDOLASE COMPLEXED WITH FRUCTOSE 1,6-BISPHOSPHATE== | |
| - | + | <StructureSection load='4ald' size='340' side='right' caption='[[4ald]], [[Resolution|resolution]] 2.80Å' scene=''> | |
| - | + | == Structural highlights == | |
| + | <table><tr><td colspan='2'>[[4ald]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. The February 2004 RCSB PDB [http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''The Glycolytic Enzymes'' by David S. Goodsell is [http://dx.doi.org/10.2210/rcsb_pdb/mom_2004_2 10.2210/rcsb_pdb/mom_2004_2]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4ALD OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4ALD FirstGlance]. <br> | ||
| + | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=2FP:1,6-FRUCTOSE+DIPHOSPHATE+(LINEAR+FORM)'>2FP</scene></td></tr> | ||
| + | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Fructose-bisphosphate_aldolase Fructose-bisphosphate aldolase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=4.1.2.13 4.1.2.13] </span></td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4ald FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4ald OCA], [http://www.rcsb.org/pdb/explore.do?structureId=4ald RCSB], [http://www.ebi.ac.uk/pdbsum/4ald PDBsum]</span></td></tr> | ||
| + | </table> | ||
| + | == Disease == | ||
| + | [[http://www.uniprot.org/uniprot/ALDOA_HUMAN ALDOA_HUMAN]] Defects in ALDOA are the cause of glycogen storage disease type 12 (GSD12) [MIM:[http://omim.org/entry/611881 611881]]; also known as red cell aldolase deficiency. A metabolic disorder associated with increased hepatic glycogen and hemolytic anemia. It may lead to myopathy with exercise intolerance and rhabdomyolysis.<ref>PMID:14766013</ref> <ref>PMID:2825199</ref> <ref>PMID:2229018</ref> <ref>PMID:8598869</ref> <ref>PMID:14615364</ref> | ||
| + | == Function == | ||
| + | [[http://www.uniprot.org/uniprot/ALDOA_HUMAN ALDOA_HUMAN]] Plays a key role in glycolysis and gluconeogenesis. In addition, may also function as scaffolding protein (By similarity). | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/al/4ald_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | Fructose 1,6-bisphosphate aldolase catalyzes the reversible cleavage of fructose 1,6-bisphosphate and fructose 1-phosphate to dihydroxyacetone phosphate and either glyceraldehyde 3-phosphate or glyceraldehyde, respectively. Catalysis involves the formation of a Schiff's base intermediate formed at the epsilon-amino group of Lys229. The existing apo-enzyme structure was refined using the crystallographic free-R-factor and maximum likelihood methods that have been shown to give improved structural results that are less subject to model bias. Crystals were also soaked with the natural substrate (fructose 1,6-bisphosphate), and the crystal structure of this complex has been determined to 2.8 A. The apo structure differs from the previous Brookhaven-deposited structure (1ald) in the flexible C-terminal region. This is also the region where the native and complex structures exhibit differences. The conformational changes between native and complex structure are not large, but the observed complex does not involve the full formation of the Schiff's base intermediate, and suggests a preliminary hydrogen-bonded Michaelis complex before the formation of the covalent complex. | ||
| - | + | Crystal structure of human muscle aldolase complexed with fructose 1,6-bisphosphate: mechanistic implications.,Dalby A, Dauter Z, Littlechild JA Protein Sci. 1999 Feb;8(2):291-7. PMID:10048322<ref>PMID:10048322</ref> | |
| - | + | ||
| - | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | + | </div> | |
| - | + | ||
| - | + | ||
| - | + | ||
==See Also== | ==See Also== | ||
*[[Aldolase|Aldolase]] | *[[Aldolase|Aldolase]] | ||
*[[Fructose Bisphosphate Aldolase|Fructose Bisphosphate Aldolase]] | *[[Fructose Bisphosphate Aldolase|Fructose Bisphosphate Aldolase]] | ||
| - | + | == References == | |
| - | == | + | <references/> |
| - | + | __TOC__ | |
| + | </StructureSection> | ||
[[Category: Fructose-bisphosphate aldolase]] | [[Category: Fructose-bisphosphate aldolase]] | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: RCSB PDB Molecule of the Month]] | [[Category: RCSB PDB Molecule of the Month]] | ||
[[Category: The Glycolytic Enzymes]] | [[Category: The Glycolytic Enzymes]] | ||
| - | [[Category: Dalby, A R | + | [[Category: Dalby, A R]] |
| - | [[Category: Dauter, Z | + | [[Category: Dauter, Z]] |
| - | [[Category: Littlechild, J A | + | [[Category: Littlechild, J A]] |
[[Category: Complex]] | [[Category: Complex]] | ||
[[Category: Lyase]] | [[Category: Lyase]] | ||
[[Category: Tim barrel]] | [[Category: Tim barrel]] | ||
[[Category: Type 1 aldolase]] | [[Category: Type 1 aldolase]] | ||
Revision as of 09:33, 21 December 2014
HUMAN MUSCLE FRUCTOSE 1,6-BISPHOSPHATE ALDOLASE COMPLEXED WITH FRUCTOSE 1,6-BISPHOSPHATE
| |||||||||||