1s5o
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Image:1s5o.gif|left|200px]] | + | [[Image:1s5o.gif|left|200px]] |
| - | + | ||
| - | '''Structural and Mutational Characterization of L-carnitine Binding to Human carnitine Acetyltransferase''' | + | {{Structure |
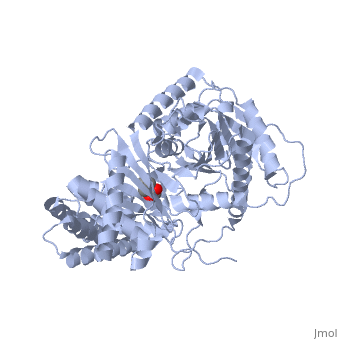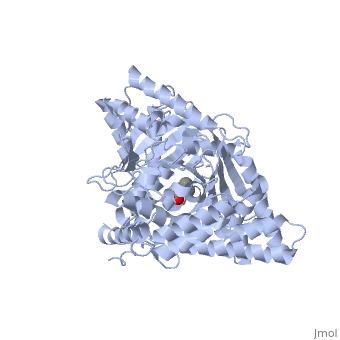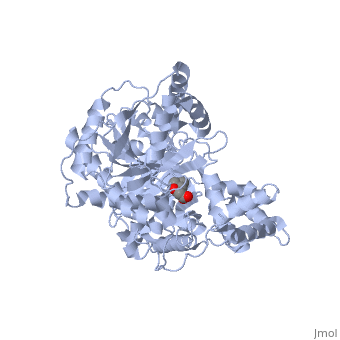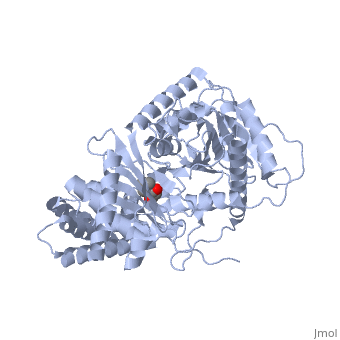
| + | |PDB= 1s5o |SIZE=350|CAPTION= <scene name='initialview01'>1s5o</scene>, resolution 1.8Å | ||
| + | |SITE= | ||
| + | |LIGAND= <scene name='pdbligand=152:CARNITINE'>152</scene> | ||
| + | |ACTIVITY= [http://en.wikipedia.org/wiki/Carnitine_O-acetyltransferase Carnitine O-acetyltransferase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.3.1.7 2.3.1.7] | ||
| + | |GENE= | ||
| + | }} | ||
| + | |||
| + | '''Structural and Mutational Characterization of L-carnitine Binding to Human carnitine Acetyltransferase''' | ||
| + | |||
==Overview== | ==Overview== | ||
| Line 10: | Line 19: | ||
==About this Structure== | ==About this Structure== | ||
| - | 1S5O is a [ | + | 1S5O is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1S5O OCA]. |
==Reference== | ==Reference== | ||
| - | Structural and mutational characterization of L-carnitine binding to human carnitine acetyltransferase., Govindasamy L, Kukar T, Lian W, Pedersen B, Gu Y, Agbandje-McKenna M, Jin S, McKenna R, Wu D, J Struct Biol. 2004 Jun;146(3):416-24. PMID:[http:// | + | Structural and mutational characterization of L-carnitine binding to human carnitine acetyltransferase., Govindasamy L, Kukar T, Lian W, Pedersen B, Gu Y, Agbandje-McKenna M, Jin S, McKenna R, Wu D, J Struct Biol. 2004 Jun;146(3):416-24. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/15099582 15099582] |
[[Category: Carnitine O-acetyltransferase]] | [[Category: Carnitine O-acetyltransferase]] | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
| Line 29: | Line 38: | ||
[[Category: binary complex]] | [[Category: binary complex]] | ||
[[Category: carnitine acetyltransferase]] | [[Category: carnitine acetyltransferase]] | ||
| - | [[Category: steady-state enzyme | + | [[Category: steady-state enzyme kinetic]] |
[[Category: substrate binding site]] | [[Category: substrate binding site]] | ||
[[Category: x-ray structure]] | [[Category: x-ray structure]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 14:00:49 2008'' |
Revision as of 12:00, 20 March 2008
| |||||||
| , resolution 1.8Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Activity: | Carnitine O-acetyltransferase, with EC number 2.3.1.7 | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Structural and Mutational Characterization of L-carnitine Binding to Human carnitine Acetyltransferase
Contents |
Overview
We report the crystal structure of a binary complex of human peroxisomal carnitine acetyltransferase and the substrate l-carnitine, refined to a resolution of 1.8 Angstrom with an R(factor) value of 18.9% (R(free)=22.3%). L-carnitine binds to a preformed pocket in the active site tunnel of carnitine acetyltransferase aligned with His(322). The quaternary nitrogen of carnitine forms a pi-cation interaction with Phe(545), while Arg(497) forms an electrostatic interaction with the negatively charged carboxylate group. An extensive hydrogen bond network also occurs between the carboxylate group and Tyr(431), Thr(444), and a bound water molecule. Site-directed mutagenesis and kinetic characterization reveals that Tyr(431), Thr(444), Arg(497), and Phe(545) are essential for high affinity binding of L-carnitine.
Disease
Known disease associated with this structure: Carnitine acetyltransferase deficiency (1) OMIM:[600184]
About this Structure
1S5O is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Structural and mutational characterization of L-carnitine binding to human carnitine acetyltransferase., Govindasamy L, Kukar T, Lian W, Pedersen B, Gu Y, Agbandje-McKenna M, Jin S, McKenna R, Wu D, J Struct Biol. 2004 Jun;146(3):416-24. PMID:15099582
Page seeded by OCA on Thu Mar 20 14:00:49 2008
Categories: Carnitine O-acetyltransferase | Homo sapiens | Single protein | Agbandje-Mckenna, M. | Govindasamy, L. | Gu, Y. | Jin, S. | Kukar, T. | Lian, W. | Mckenna, R. | Pedersen, B. | Wu, D. | 152 | Binary complex | Carnitine acetyltransferase | Steady-state enzyme kinetic | Substrate binding site | X-ray structure