This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Human lactoferrin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='1dsn' size='450' side='right' scene='' caption='Human lactoferrin complex with Fe and carbonate, [[1dsn]]'> | |
| - | {{STRUCTURE_1dsn|PDB=1dsn|SCENE=}} | ||
=Amino-Terminal Half-Molecule of Human Lactoferrin= | =Amino-Terminal Half-Molecule of Human Lactoferrin= | ||
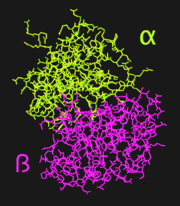
Human lactoferrin, LF, is a protein in the transferrin family. As such, it has the ability to tightly bind iron in conjunction with a large-scale conformational change associated with iron binding and release.<ref name="faber">PMID:8594202</ref> These properties give lactoferrin the ability to regulate iron, and possibly other metal, ion levels in the fluids and secretions, such as milk, of animals.<ref name="faber" /> Lactoferrin is folded into two lobes: the N-terminal half, LF<sub>N</sub> ([[1dsn]]), and the C-terminal half, LF<sub>C</sub>. The two LF lobes have 37% homology and very similar tertiary structures; it has been suggested that the two lobes are the product of gene duplication.<ref name="farnaud" /> Each lobe of LF<sub>N</sub> is further subdivided into two similarly sized α and β domains (Figure 1); the <scene name='Sandbox_Reserved_302/Ligand_site/1'>iron binding site</scene> is situated in a deep cleft between the two domains.<ref name="faber" /> | Human lactoferrin, LF, is a protein in the transferrin family. As such, it has the ability to tightly bind iron in conjunction with a large-scale conformational change associated with iron binding and release.<ref name="faber">PMID:8594202</ref> These properties give lactoferrin the ability to regulate iron, and possibly other metal, ion levels in the fluids and secretions, such as milk, of animals.<ref name="faber" /> Lactoferrin is folded into two lobes: the N-terminal half, LF<sub>N</sub> ([[1dsn]]), and the C-terminal half, LF<sub>C</sub>. The two LF lobes have 37% homology and very similar tertiary structures; it has been suggested that the two lobes are the product of gene duplication.<ref name="farnaud" /> Each lobe of LF<sub>N</sub> is further subdivided into two similarly sized α and β domains (Figure 1); the <scene name='Sandbox_Reserved_302/Ligand_site/1'>iron binding site</scene> is situated in a deep cleft between the two domains.<ref name="faber" /> | ||
Revision as of 14:10, 21 December 2014
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Alexander Berchansky, Michal Harel, Andrea Gorrell