This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Human lactoferrin
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
=Structure= | =Structure= | ||
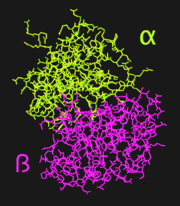
[[Image:1DSN_Domains.png|left|thumb|'''Figure 1.''' Cartoon illustrating the alpha (green) and beta (magenta) domains of LF<sub>N</sub>]] | [[Image:1DSN_Domains.png|left|thumb|'''Figure 1.''' Cartoon illustrating the alpha (green) and beta (magenta) domains of LF<sub>N</sub>]] | ||
| + | {{Clear}} | ||
The amino-terminal half-molecule of human lactoferrin (LF<sub>N</sub>) is comprised of a single 333 amino acid chain divided into two similarly-sized α and β domains. The iron binding site is located within a deep cleft between the lobes, where iron is bound by <scene name='Sandbox_Reserved_302/Helix_3_and_5/2'>Helices 3 and 5</scene> of the α and β domains, respectively. Iron, which is bound to a carboxylate ion, is bound by Asp60, Ala123, and Gly124. Although unwound in LF<sub>N</sub>, <scene name='Sandbox_Reserved_302/Pigtail/1'>residues 313 to 333</scene> form a helix when joined to LF<sub>C</sub>, forming the full LF protein.<ref name="faber" /> | The amino-terminal half-molecule of human lactoferrin (LF<sub>N</sub>) is comprised of a single 333 amino acid chain divided into two similarly-sized α and β domains. The iron binding site is located within a deep cleft between the lobes, where iron is bound by <scene name='Sandbox_Reserved_302/Helix_3_and_5/2'>Helices 3 and 5</scene> of the α and β domains, respectively. Iron, which is bound to a carboxylate ion, is bound by Asp60, Ala123, and Gly124. Although unwound in LF<sub>N</sub>, <scene name='Sandbox_Reserved_302/Pigtail/1'>residues 313 to 333</scene> form a helix when joined to LF<sub>C</sub>, forming the full LF protein.<ref name="faber" /> | ||
| Line 23: | Line 24: | ||
[[Lactoferrin]] | [[Lactoferrin]] | ||
| - | + | </StructureSection> | |
=References= | =References= | ||
<references /> | <references /> | ||
Page originally authored by Christian Axen | Page originally authored by Christian Axen | ||
Revision as of 14:12, 21 December 2014
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Faber HR, Bland T, Day CL, Norris GE, Tweedie JW, Baker EN. Altered domain closure and iron binding in transferrins: the crystal structure of the Asp60Ser mutant of the amino-terminal half-molecule of human lactoferrin. J Mol Biol. 1996 Feb 23;256(2):352-63. PMID:8594202
- ↑ 2.0 2.1 2.2 Farnaud S, Evans RW. Lactoferrin--a multifunctional protein with antimicrobial properties. Mol Immunol. 2003 Nov;40(7):395-405. PMID:14568385
- ↑ 3.0 3.1 3.2 Sanchez L, Calvo M, Brock JH. Biological role of lactoferrin. Arch Dis Child. 1992 May;67(5):657-61. PMID:1599309
- ↑ Gerstein M, Anderson BF, Norris GE, Baker EN, Lesk AM, Chothia C. Domain closure in lactoferrin. Two hinges produce a see-saw motion between alternative close-packed interfaces. J Mol Biol. 1993 Nov 20;234(2):357-72. PMID:8230220 doi:http://dx.doi.org/10.1006/jmbi.1993.1592
- ↑ 5.0 5.1 van der Strate BW, Beljaars L, Molema G, Harmsen MC, Meijer DK. Antiviral activities of lactoferrin. Antiviral Res. 2001 Dec;52(3):225-39. PMID:11675140
Page originally authored by Christian Axen
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Alexander Berchansky, Michal Harel, Andrea Gorrell