Sandbox Reserved 960
From Proteopedia
(Difference between revisions)
| Line 52: | Line 52: | ||
Furthermore, non covalent bonds also play an important role. | Furthermore, non covalent bonds also play an important role. | ||
| - | Indeed, at pH 5.5, among the numerous other, two hydrogen bonds are particularly noticeable because of their importance in the formation of the loop stabilizing H4. <scene name='60/604479/Hydrogene_bond_1/2'> | + | Indeed, at pH 5.5, among the numerous other, two hydrogen bonds are particularly noticeable because of their importance in the formation of the loop stabilizing <scene name='60/604479/H4/1'>H4</scene>. <scene name='60/604479/Hydrogene_bond_1/2'>The first bond</scene> is established by Asp 66 and Leu 58 whereas <scene name='60/604479/Hydrogene_bond_2/1'>the second</scene> is formed between Asp 60 and Ala 63. |
=== Cavity === | === Cavity === | ||
| Line 65: | Line 65: | ||
== Ligands == | == Ligands == | ||
[[Image:CMJ_Ligplot.png|150px|right|thumb|'''Fig.2''' CMJ Ligplot]] | [[Image:CMJ_Ligplot.png|150px|right|thumb|'''Fig.2''' CMJ Ligplot]] | ||
| - | In order to determine this {{Template:ColorKey Composition Protein}}’s structure, several{{Template:ColorKey Composition Ligand}} has been used at pH 5.5 because this low pH fits with its natural medium in the bee antenna. | + | In order to determine this {{Template:ColorKey Composition Protein}}’s structure, several {{Template:ColorKey Composition Ligand}} has been used at pH 5.5 because this low pH fits with its natural medium in the bee antenna. |
The three ligands used to characterize and purify AmelASP1 are : | The three ligands used to characterize and purify AmelASP1 are : | ||
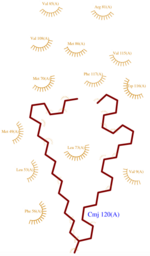
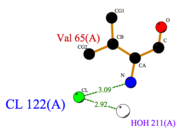
*<scene name='60/604479/Cmj/3'>CMJ</scene> also known as (20s)-20-Methyldotetracontane, is a serendipitous ligand. This term signify that the purification of this molecule was completely fortuitous. It is a big unsaturated mono-methyl branched carbone chain with formula C43H88. This ligand fits in the {{Template:ColorKey_Hydrophobic}} cavity of AmelASP1 thanks to several interactions with <scene name='60/604479/Cmj_binding_residues/2'>specific residues.</scene> | *<scene name='60/604479/Cmj/3'>CMJ</scene> also known as (20s)-20-Methyldotetracontane, is a serendipitous ligand. This term signify that the purification of this molecule was completely fortuitous. It is a big unsaturated mono-methyl branched carbone chain with formula C43H88. This ligand fits in the {{Template:ColorKey_Hydrophobic}} cavity of AmelASP1 thanks to several interactions with <scene name='60/604479/Cmj_binding_residues/2'>specific residues.</scene> | ||
Revision as of 23:32, 23 December 2014
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
Crystal structure of the Antennal Specific Protein-1 from Apis mellifera (AmelASP1) with a serendipitous ligand at pH 5.5
| |||||||||||
Contributors
Sophie Morin & Mathias Buytaert
References for further information on the pheromone binding protein from Apis mellifera
- ↑ Pesenti ME, Spinelli S, Bezirard V, Briand L, Pernollet JC, Tegoni M, Cambillau C. Structural basis of the honey bee PBP pheromone and pH-induced conformational change. J Mol Biol. 2008 Jun 27;380(1):158-69. Epub 2008 Apr 27. PMID:18508083 doi:10.1016/j.jmb.2008.04.048
- ↑ Pesenti ME, Spinelli S, Bezirard V, Briand L, Pernollet JC, Campanacci V, Tegoni M, Cambillau C. Queen bee pheromone binding protein pH-induced domain swapping favors pheromone release. J Mol Biol. 2009 Jul 31;390(5):981-90. Epub 2009 May 28. PMID:19481550 doi:10.1016/j.jmb.2009.05.067
- ↑ Han L, Zhang YJ, Zhang L, Cui X, Yu J, Zhang Z, Liu MS. Operating mechanism and molecular dynamics of pheromone-binding protein ASP1 as influenced by pH. PLoS One. 2014 Oct 22;9(10):e110565. doi: 10.1371/journal.pone.0110565., eCollection 2014. PMID:25337796 doi:http://dx.doi.org/10.1371/journal.pone.0110565
- ↑ Lartigue A, Gruez A, Briand L, Blon F, Bezirard V, Walsh M, Pernollet JC, Tegoni M, Cambillau C. Sulfur single-wavelength anomalous diffraction crystal structure of a pheromone-binding protein from the honeybee Apis mellifera L. J Biol Chem. 2004 Feb 6;279(6):4459-64. Epub 2003 Oct 31. PMID:14594955 doi:10.1074/jbc.M311212200
- ↑ http://www.genome.jp/dbget-bin/www_bget?pdb:3FE6