We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
3frp
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3frp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3frp OCA], [http://www.rcsb.org/pdb/explore.do?structureId=3frp RCSB], [http://www.ebi.ac.uk/pdbsum/3frp PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3frp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3frp OCA], [http://www.rcsb.org/pdb/explore.do?structureId=3frp RCSB], [http://www.ebi.ac.uk/pdbsum/3frp PDBsum]</span></td></tr> | ||
</table> | </table> | ||
| + | == Function == | ||
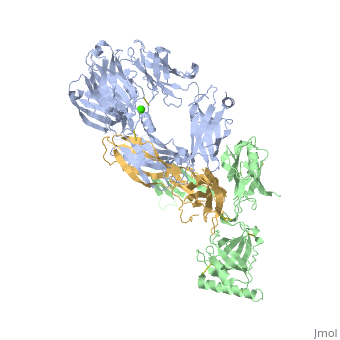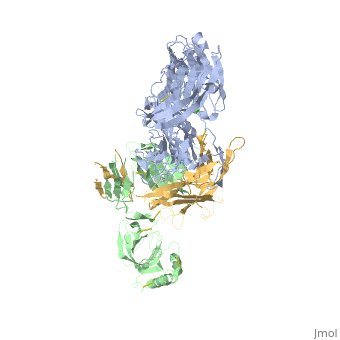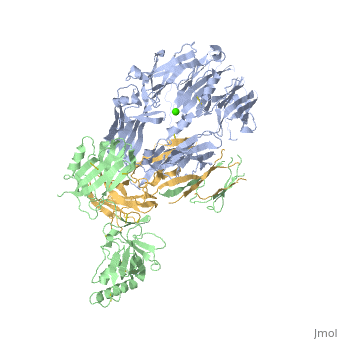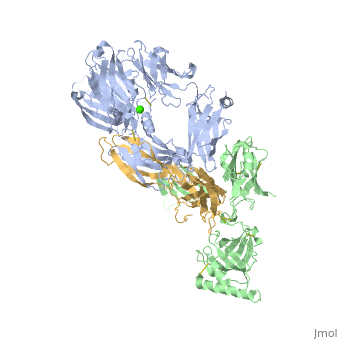
| + | [[http://www.uniprot.org/uniprot/CO3_NAJKA CO3_NAJKA]] Complement-activating protein in cobra venom. It is a structural and functional analog of complement component C3b, the activated form of C3. It binds factor B (CFB), which is subsequently cleaved by factor D (CFD) to form the bimolecular complex CVF/Bb. CVF/Bb is a C3/C5 convertase that cleaves both complement components C3 and C5. Structurally, it resembles the C3b degradation product C3c, which is not able to form a C3/C5 convertase. Unlike C3b/Bb, CVF/Bb is a stable complex and completely resistant to the actions of complement regulatory factors H (CFH) and I (CFI). Therefore, CVF continuously activates complement resulting in the depletion of complement activity. | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
Revision as of 08:23, 25 December 2014
Crystal Structure of Cobra Venom Factor, a Co-factor for C3- and C5 convertase CVFBb
| |||||||||||
Categories: Naja kaouthia | Krishnan, V | Narayana, S V.L | Cobra venom factor | Complement alternate pathway | Complement c3 and c5 convertase cvfbb | Complement pathway | Complement protein | Glycoprotein | Hydrolase cofactor | Immune response | Inflammatory response | Innate immunity | Naja naja kouthia | Secreted | Thioester bond | Toxin