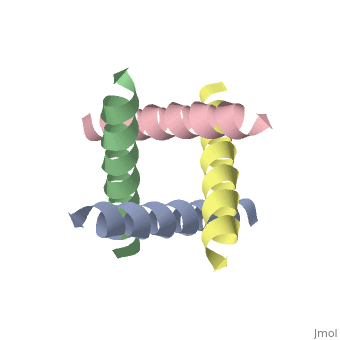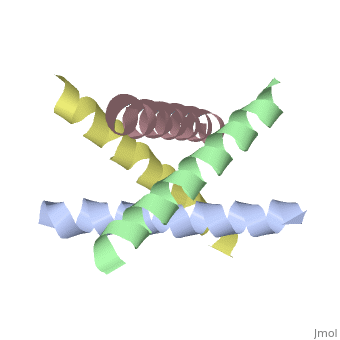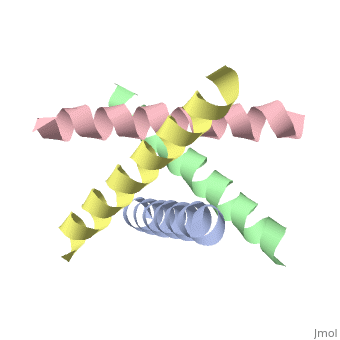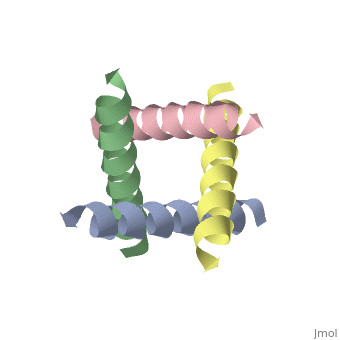We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
1nyj
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1nyj FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1nyj OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1nyj RCSB], [http://www.ebi.ac.uk/pdbsum/1nyj PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1nyj FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1nyj OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1nyj RCSB], [http://www.ebi.ac.uk/pdbsum/1nyj PDBsum]</span></td></tr> | ||
</table> | </table> | ||
| + | == Function == | ||
| + | [[http://www.uniprot.org/uniprot/M2_I77AB M2_I77AB]] Forms a proton-selective ion channel that is necessary for the efficient release of the viral genome during virus entry. After attaching to the cell surface, the virion enters the cell by endocytosis. Acidification of the endosome triggers M2 ion channel activity. The influx of protons into virion interior is believed to disrupt interactions between the viral ribonucleoprotein (RNP), matrix protein 1 (M1), and lipid bilayers, thereby freeing the viral genome from interaction with viral proteins and enabling RNA segments to migrate to the host cell nucleus, where influenza virus RNA transcription and replication occur. Also plays a role in viral proteins secretory pathway. Elevates the intravesicular pH of normally acidic compartments, such as trans-Golgi network, preventing newly formed hemagglutinin from premature switching to the fusion-active conformation (By similarity). | ||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
Revision as of 15:42, 25 December 2014
The closed state structure of M2 protein H+ channel by solid state NMR spectroscopy
| |||||||||||