We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 959
From Proteopedia
(Difference between revisions)
| Line 53: | Line 53: | ||
==== Monomere structure ==== | ==== Monomere structure ==== | ||
| - | Each defensin monomer consists of three strands of antiparallel β-sheet incorporating 60% of the residues. Two β-turns and three disulfide bonds add further restrictions to the conformational freedom of the monomer. | + | Each defensin monomer consists of three strands of antiparallel β-sheet incorporating 60% of the residues. Two β-turns and three disulfide bonds add further restrictions to the conformational freedom of the monomer. |
==== Dimere structure ==== | ==== Dimere structure ==== | ||
Revision as of 18:27, 27 December 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Introduction
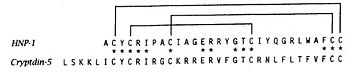
Defensins (DEF) are a family of proteins which are involved in host defense in the epithelia of mucosal surfaces such as those of the intestin, respiratory tract, urinary tract, and vagina. They are antimicrobial and cytotoxic. All the protein of the family are distinguished by a cystein motif and are encoded on the chromozome 8.[1]
There are many defensin but in this article we'll focus on the defensin-α-1. It is a polypeptide which is found in the microbicidal granules of neutrophils. It's syntetisize in the paneth cell, which plays a role in the defense process. defensin-α-1 plays a particular role in phagocite-mediated host defense. [2]
| |||||||||||