We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 959
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
<div class="center" style="width: auto; margin-left: auto; margin-right: auto;"> | <div class="center" style="width: auto; margin-left: auto; margin-right: auto;"> | ||
| - | + | <big>'''Defensins-α-1'''</big> | |
</div> | </div> | ||
| Line 16: | Line 16: | ||
<StructureSection load='2pm4' size='450' side='right'caption='Crystal Structure of Defensin, (PDB code [[2pm4]]) '> | <StructureSection load='2pm4' size='450' side='right'caption='Crystal Structure of Defensin, (PDB code [[2pm4]]) '> | ||
| - | + | == Biological role == | |
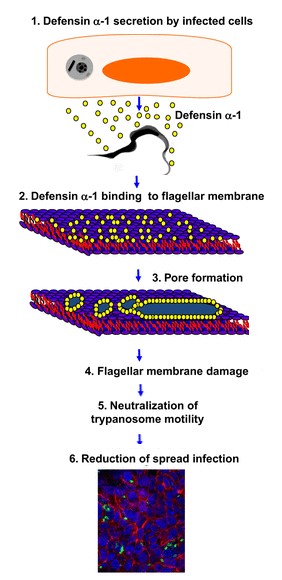
The presence of an unknown microbial cell active the transcription of the defensin-α-1 gene. When the RNAm is translated defensins-α-1 are not active. They became active in the Golgi apparatus, in which they are biologically cleaved. Then they are stock in the azurophil granule lumen. After the exocytosis, two defensins-α-1 create a dimere, which'll attack the membran of the microbial cell, by the formation of channel. | The presence of an unknown microbial cell active the transcription of the defensin-α-1 gene. When the RNAm is translated defensins-α-1 are not active. They became active in the Golgi apparatus, in which they are biologically cleaved. Then they are stock in the azurophil granule lumen. After the exocytosis, two defensins-α-1 create a dimere, which'll attack the membran of the microbial cell, by the formation of channel. | ||
This mecanism is sum up in the following picture. | This mecanism is sum up in the following picture. | ||
| Line 29: | Line 29: | ||
| - | + | == Structure == | |
| - | + | === Monomere structure === | |
Defensin-α-1 peptides are cationic antimicrobial peptides that are synthesized in vivo as inactive precursors. <br /> | Defensin-α-1 peptides are cationic antimicrobial peptides that are synthesized in vivo as inactive precursors. <br /> | ||
| Line 51: | Line 51: | ||
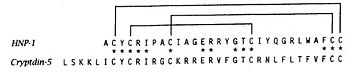
Each defensin monomer consists of three strands of antiparallel β-sheet incorporating 60% of the residues. Two β-turns and three disulfide bonds add further restrictions to the conformational freedom of the monomer.<ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2049026/</ref>. | Each defensin monomer consists of three strands of antiparallel β-sheet incorporating 60% of the residues. Two β-turns and three disulfide bonds add further restrictions to the conformational freedom of the monomer.<ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2049026/</ref>. | ||
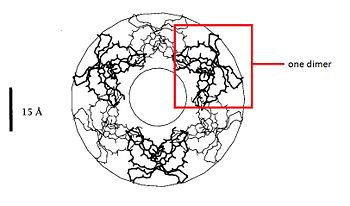
| - | + | === Dimere structure === | |
The dimere is formed by joining identical β-strands of the two monomers together to create a symmetrical six-stranded P-sheet. This extended β-sheet twists and curls to form a basket-shaped structure that has a small solvent-accessible channel passing through it. The base of the basket is hydrophobic while the top, which contains the N- and C-terminal domains of the two defensin monomers, is polar. This dimer-of-dimers may be an essential feature of defensins’interaction with membranes.<ref>Gary Fujii, Michael E.Selsted, David Eisenberg. Defensins promote fusion and lysis of negatively charged membranes. Protein Science. 1993. Cambridge University.</ref> | The dimere is formed by joining identical β-strands of the two monomers together to create a symmetrical six-stranded P-sheet. This extended β-sheet twists and curls to form a basket-shaped structure that has a small solvent-accessible channel passing through it. The base of the basket is hydrophobic while the top, which contains the N- and C-terminal domains of the two defensin monomers, is polar. This dimer-of-dimers may be an essential feature of defensins’interaction with membranes.<ref>Gary Fujii, Michael E.Selsted, David Eisenberg. Defensins promote fusion and lysis of negatively charged membranes. Protein Science. 1993. Cambridge University.</ref> | ||
| - | + | === Binding with the cell membrane === | |
The interaction of defensin-α-1 with cell membranes involves single dimer binding electrostatically to the cell surface. | The interaction of defensin-α-1 with cell membranes involves single dimer binding electrostatically to the cell surface. | ||
In fact, the hydrophobic basket bottom of the defensin-α-1 dimer is inserted into the the hydrocarbon layer of one lipid monolayer while the polar group of the basket top and the <scene name='60/604478/Arg/1'>arginine</scene> maintain contact with the headgroup and aqueaus phase. Two dimers created a channel. | In fact, the hydrophobic basket bottom of the defensin-α-1 dimer is inserted into the the hydrocarbon layer of one lipid monolayer while the polar group of the basket top and the <scene name='60/604478/Arg/1'>arginine</scene> maintain contact with the headgroup and aqueaus phase. Two dimers created a channel. | ||
| Line 66: | Line 66: | ||
|} | |} | ||
| - | === Example === | + | ==== Example ==== |
DEF are known to play a role in the in the initiation of innate immune responses to some microbial pathogens. They are antimicrobial peptides of innate immunity functioning by non-specific binding to anionic phospholipids in bacterial membranes. For example defensins-α-1 have a role against the bacteria ''Trypanosoma cruzi''. <br /><ref>http://iai.asm.org/content/81/11/4139.full/ref</ref>.''Trypanosoma cruzy'' or ''Cruzy'' debilitats Chagas disease, which affects millions of people and products significant morbidity and mortality. The defensine-α-1 are secreted by the HCT116 cells (which are Paneth cells), when they are infect by ''Cruzy''. They reduces the infection making damage of the flagella structure. This damage inhibit parasite motility and reduce cellular infection. This reaction is introduce in the following drawing. | DEF are known to play a role in the in the initiation of innate immune responses to some microbial pathogens. They are antimicrobial peptides of innate immunity functioning by non-specific binding to anionic phospholipids in bacterial membranes. For example defensins-α-1 have a role against the bacteria ''Trypanosoma cruzi''. <br /><ref>http://iai.asm.org/content/81/11/4139.full/ref</ref>.''Trypanosoma cruzy'' or ''Cruzy'' debilitats Chagas disease, which affects millions of people and products significant morbidity and mortality. The defensine-α-1 are secreted by the HCT116 cells (which are Paneth cells), when they are infect by ''Cruzy''. They reduces the infection making damage of the flagella structure. This damage inhibit parasite motility and reduce cellular infection. This reaction is introduce in the following drawing. | ||
Revision as of 18:31, 29 December 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Defensins-α-1
Introduction
Defensins (DEF) are a family of proteins which are involved in host defense in the epithelia of mucosal surfaces such as those of the intestin, respiratory tract, urinary tract, and vagina. They are antimicrobial and cytotoxic. All the protein of the family are distinguished by a cystein motif and are encoded on the chromozome 8.[1]
There are many defensin but in this article we'll focus on the defensin-α-1. It is a polypeptide which is found in the microbicidal granules of neutrophils. It's syntetisize in the paneth cell, which plays a role in the defense process. defensin-α-1 plays a particular role in phagocite-mediated host defense. [2]
| |||||||||||