This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
2g2u
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Image:2g2u.gif|left|200px]] | + | [[Image:2g2u.gif|left|200px]] |
| - | + | ||
| - | '''Crystal Structure of the SHV-1 Beta-lactamase/Beta-lactamase inhibitor protein (BLIP) complex''' | + | {{Structure |
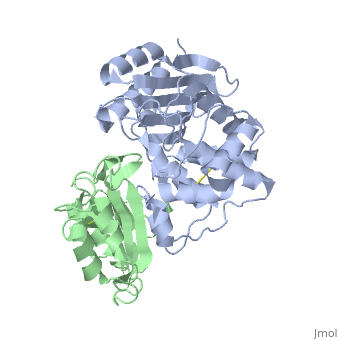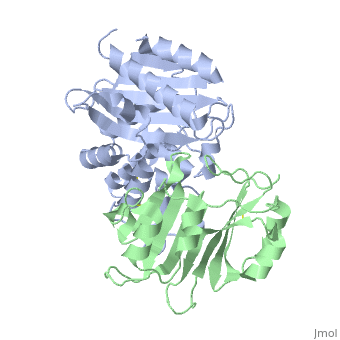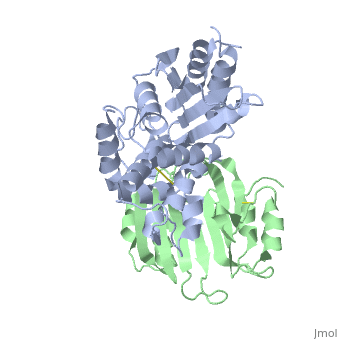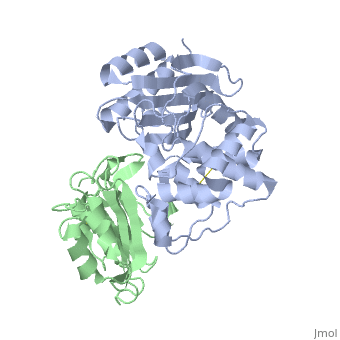
| + | |PDB= 2g2u |SIZE=350|CAPTION= <scene name='initialview01'>2g2u</scene>, resolution 1.60Å | ||
| + | |SITE= | ||
| + | |LIGAND= | ||
| + | |ACTIVITY= [http://en.wikipedia.org/wiki/Beta-lactamase Beta-lactamase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.5.2.6 3.5.2.6] | ||
| + | |GENE= bla, shv1 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=573 Klebsiella pneumoniae]) | ||
| + | }} | ||
| + | |||
| + | '''Crystal Structure of the SHV-1 Beta-lactamase/Beta-lactamase inhibitor protein (BLIP) complex''' | ||
| + | |||
==Overview== | ==Overview== | ||
| Line 7: | Line 16: | ||
==About this Structure== | ==About this Structure== | ||
| - | 2G2U is a [ | + | 2G2U is a [[Protein complex]] structure of sequences from [http://en.wikipedia.org/wiki/Klebsiella_pneumoniae Klebsiella pneumoniae] and [http://en.wikipedia.org/wiki/Streptomyces_clavuligerus Streptomyces clavuligerus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2G2U OCA]. |
==Reference== | ==Reference== | ||
| - | Structural and computational characterization of the SHV-1 beta-lactamase-beta-lactamase inhibitor protein interface., Reynolds KA, Thomson JM, Corbett KD, Bethel CR, Berger JM, Kirsch JF, Bonomo RA, Handel TM, J Biol Chem. 2006 Sep 8;281(36):26745-53. Epub 2006 Jun 29. PMID:[http:// | + | Structural and computational characterization of the SHV-1 beta-lactamase-beta-lactamase inhibitor protein interface., Reynolds KA, Thomson JM, Corbett KD, Bethel CR, Berger JM, Kirsch JF, Bonomo RA, Handel TM, J Biol Chem. 2006 Sep 8;281(36):26745-53. Epub 2006 Jun 29. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/16809340 16809340] |
[[Category: Beta-lactamase]] | [[Category: Beta-lactamase]] | ||
[[Category: Klebsiella pneumoniae]] | [[Category: Klebsiella pneumoniae]] | ||
| Line 29: | Line 38: | ||
[[Category: shv-1]] | [[Category: shv-1]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 16:59:43 2008'' |
Revision as of 14:59, 20 March 2008
| |||||||
| , resolution 1.60Å | |||||||
|---|---|---|---|---|---|---|---|
| Gene: | bla, shv1 (Klebsiella pneumoniae) | ||||||
| Activity: | Beta-lactamase, with EC number 3.5.2.6 | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Crystal Structure of the SHV-1 Beta-lactamase/Beta-lactamase inhibitor protein (BLIP) complex
Overview
Beta-lactamase inhibitor protein (BLIP) binds a variety of class A beta-lactamases with affinities ranging from micromolar to picomolar. Whereas the TEM-1 and SHV-1 beta-lactamases are almost structurally identical, BLIP binds TEM-1 approximately 1000-fold tighter than SHV-1. Determining the underlying source of this affinity difference is important for understanding the molecular basis of beta-lactamase inhibition and mechanisms of protein-protein interface specificity and affinity. Here we present the 1.6A resolution crystal structure of SHV-1.BLIP. In addition, a point mutation was identified, SHV D104E, that increases SHV.BLIP binding affinity from micromolar to nanomolar. Comparison of the SHV-1.BLIP structure with the published TEM-1.BLIP structure suggests that the increased volume of Glu-104 stabilizes a key binding loop in the interface. Solution of the 1.8A SHV D104K.BLIP crystal structure identifies a novel conformation in which this binding loop is removed from the interface. Using these structural data, we evaluated the ability of EGAD, a program developed for computational protein design, to calculate changes in the stability of mutant beta-lactamase.BLIP complexes. Changes in binding affinity were calculated within an error of 1.6 kcal/mol of the experimental values for 112 mutations at the TEM-1.BLIP interface and within an error of 2.2 kcal/mol for 24 mutations at the SHV-1.BLIP interface. The reasonable success of EGAD in predicting changes in interface stability is a promising step toward understanding the stability of the beta-lactamase.BLIP complexes and computationally assisted design of tight binding BLIP variants.
About this Structure
2G2U is a Protein complex structure of sequences from Klebsiella pneumoniae and Streptomyces clavuligerus. Full crystallographic information is available from OCA.
Reference
Structural and computational characterization of the SHV-1 beta-lactamase-beta-lactamase inhibitor protein interface., Reynolds KA, Thomson JM, Corbett KD, Bethel CR, Berger JM, Kirsch JF, Bonomo RA, Handel TM, J Biol Chem. 2006 Sep 8;281(36):26745-53. Epub 2006 Jun 29. PMID:16809340
Page seeded by OCA on Thu Mar 20 16:59:43 2008