TmAFP is an hyperactive antifreeze protein and its origin is the Tenebrio Mollitor beetle.Due to TmAFP, Tenebrio Mollitor beetle has resistance against freezing - provides protection against physical and osmotic stresses.
The protein consists of 84 amino acids and the molecular weight is 8.4 kDA.
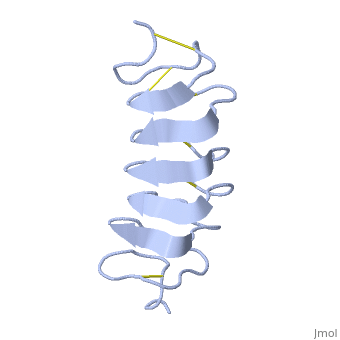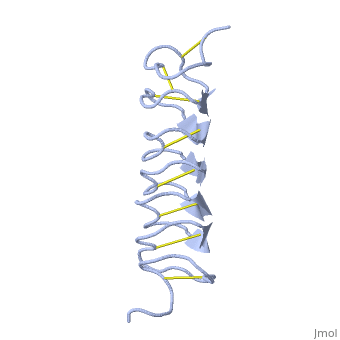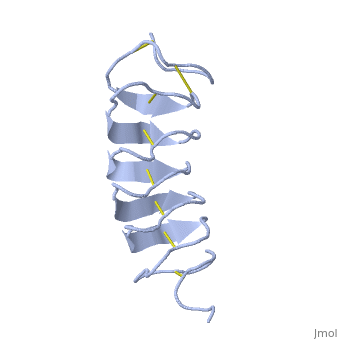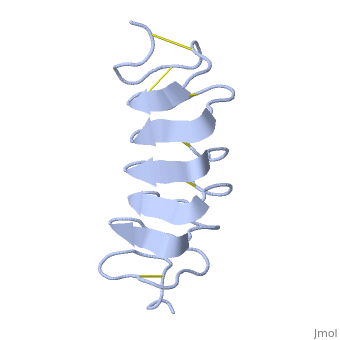
TmAFP is protein rich of threonine and cystein in form of regular parallel beta-helix. It composed of 7 tandem repeats which consist of 12 amino acids-(TCTxSxxCxxAx).
TCT (theonine, cystein, threonine) or ACT motifs are aligned to form a flat along one side of the molecule the Beta sheets right handed which are the binding site of the protein.
The rest of the tandem repeat forms the loop which enables very organized structure of the protein.
Cysteine all over the tandem repeats, are pared to provide the which contribute to the stability of the protein.
Six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other in the N-terminal region do not fit this pattern.
The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop hydrogen bond connections between backbone CO and NH groups.
TmAFP is the smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core.
The few Hydrophobic residues have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge.[1]
[2]
Function
The two dimensional arrays of Threonine side chain makes a remarkably good match to the repeated spacing between oxygen atoms in the ice lattice on the primary prism plane, and a reasonable match to the basal plane-This is why the activity of TmAFP ( Thermal hysteresis) is much higher than the acticity of AFP from Fish (5-10 celcius degrees and 1.5 celcius degrees respectively)
In solution the protein is monomeric but it crystallized as a .
The dimerization occurs along the surface of the beta sheets.
The 2 units of the dimer do not directly interact with each other, the contact between them is mediated by highly ordered ranks of water which form hydrogen bonding with Threonine residue.
Each water molecule forms two hydrogen bonds to the closer monomer and one to the distant monomer.
The distance between two adjacent waters is 4.64±0.20Å the same distance as between Threonine residues 4.64±0.23Å.
A good two dimensional match to the ice lattice including all 3 ranks of oxygen atoms (2 from Thr and 1 from water), implying that the ordered water molecules cold act as part of an ice surface and directly participate in the AFP-ice interaction.
The regular array formed by this 3 ranks of oxygen atoms can be seen as a small piece of one layer thick ice to be incorporated into a large ice lattice.
There is no need to readjust Threonine side chains, because they don’t present steric interference. [3]
Relevance
Structural highlights
3D structures of antifreeze protein
Antifreeze protein