We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 970
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
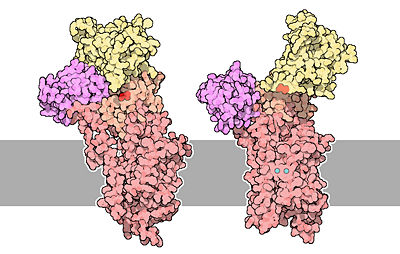
The calcium ATPase is a protein composed of 1001 aminoacids. | The calcium ATPase is a protein composed of 1001 aminoacids. | ||
| - | The protein is very rich in <scene name='60/604489/Alpha_regions/1'>alpha helices regions</scene> | + | The protein is very rich in <scene name='60/604489/Alpha_regions/1'>alpha helices regions</scene>, it contains 47% of helical regions and 16% of beta sheet regions. There are <scene name='60/604489/10_transmembrane_helices/1'>10 transmembrane alpha helices</scene>, and three of them line a channel that spans the lipid bilayer and that allows calcium to pass through membranes. It also contains too cytoplasmic loops between the transmembrane helices. When the protein is not phosphorylated, two of the transmembrane helices are disrupted and form a cavity that can bind two molecules of calcium. |
The protein is divided in <scene name='60/604489/The_4_domains_of_the_pump/1'>4 regions</scene>. The <scene name='60/604489/Transmembrane_domain/1'>transmembrane region</scene> of the protein contains the channel that span the lipid bilayer, and the calcium binding cavity. | The protein is divided in <scene name='60/604489/The_4_domains_of_the_pump/1'>4 regions</scene>. The <scene name='60/604489/Transmembrane_domain/1'>transmembrane region</scene> of the protein contains the channel that span the lipid bilayer, and the calcium binding cavity. | ||
| Line 23: | Line 23: | ||
The architecture of calcium ATPase (determined by X-Ray crystallography) allow to understand mechanisms by which the energy of ATP is coupled to the calcium transport across a membrane. | The architecture of calcium ATPase (determined by X-Ray crystallography) allow to understand mechanisms by which the energy of ATP is coupled to the calcium transport across a membrane. | ||
| - | The first step of the calcium pump catalytic cycle is the cooperative binding of <scene name='60/604489/Calcium_molecules/1'>two calcium ions</scene> in the calcium binding cavity. Then, ATP binds to the ATP binding site (nucleotide binding domain) and transfers its γ-phosphate to the <scene name='60/604489/Asp_351/1'>aspartic acide 351</scene> (phosphorylation domain). That creates a acid-stable aspartyl phosphate intermediate. The phosphorylation of Asp351 allows a large conformational changes in cytoplasmic domains: the nucleotide binding domain and the phosphorylation domain are brought into close proximity. This rearrangement causes a 90° rotation of the | + | The first step of the calcium pump catalytic cycle is the cooperative binding of <scene name='60/604489/Calcium_molecules/1'>two calcium ions</scene> in the calcium binding cavity. Then, ATP binds to the ATP binding site (nucleotide binding domain) and transfers its γ-phosphate to the <scene name='60/604489/Asp_351/1'>aspartic acide 351</scene> (phosphorylation domain). That creates a acid-stable aspartyl phosphate intermediate. The phosphorylation of Asp351 allows a large conformational changes in cytoplasmic domains: the nucleotide binding domain and the phosphorylation domain are brought into close proximity. This rearrangement causes a 90° rotation of the actuator domain, which leads to a rearrangement of the transmembrane helices. This rearrangement alters the affinity of the protein for the calcium and disrupts the calcium binding cavity. Calcium is released in the lumen of the endoplasmic reticulum/Golgi Apparatus or outside the cell. After releasing calcium, two protons bind to the transport sites (charges compensation) and the aspartyl phosphate is hydrolyzed to complete the cycle. <ref name="third">Thomas D.Pollard and William C. Earnshaw, - ''Membrane, structure and function'' - Cell Biology (second edition), p.133-136</ref> |
[[Image:51-TheCalciumPumps-calcium-pumps.jpg|400px|center|]] | [[Image:51-TheCalciumPumps-calcium-pumps.jpg|400px|center|]] | ||
Revision as of 11:56, 7 January 2015
| |||||||||||
References
- ↑ 1.0 1.1 David Goodsell, 2004 - Calcium pump molecul of the month - PDB, doi: 10.2210/rcsb_pdb/mom_2004_3
- ↑ 2.0 2.1 Benjamin Lewin, 2007 - Cells - Jones & Bartlett Learning
- ↑ 3.0 3.1 Thomas D.Pollard and William C. Earnshaw, - Membrane, structure and function - Cell Biology (second edition), p.133-136
- ↑ David H.MacLennan, William J.Rice and N. Michael Green, 1997 - The Mechanism of Ca2+ Transport by Sarco(Endo)plasmic Reticulum Ca2+-ATPases - The Journal of Biological Chemistry, p.272, 28815-28818, http://www.jbc.org/content/272/46/28815.full.html
- ↑ Marianela G.Dalghi, Marisa M.Fernández, Mariela Ferreira-Gomes, Irene C.Mangialavori, Emilio L.Malchiodi, Emanuel E.Strehler and Juan Pablo F.C.Rossi, 2013 - Plasma Membrane Calcium ATPase Activity Is Regulated by Actin Oligomers through Direct Interaction - The Journal of Biological Chemistry, p.288, 23380-23393, http://www.jbc.org/content/288/32/23380.full.
- ↑ Marisa Brini and Ernesto Carafoli, 2010 - The plasma membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium - Cold Spring Harbor Perspectives in Biology, http://cshperspectives.cshlp.org/content/3/2/a004168.full