We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 964
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
==Introduction== | ==Introduction== | ||
| - | Carbonic anhydrase II (gene name CA2), is one of fourteen | + | 1f2w is a protein from the Carbonic anhydrase II (gene name CA2) sub-sub-family, which is one of the fourteen isoforms of human α carbonic anhydrases. |
| - | + | Carbonic anhydrase II is located in the cytosol. | |
| - | 1f2w catalyzes reversible hydration of cyanamide into urea. | + | This enzyme is a lyase, which is able to break C-N links, and needs its cofactor, the zinc ion, to be activated. |
| + | |||
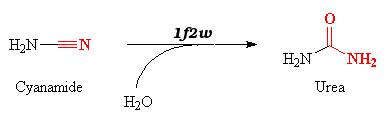
| + | The 1f2w protein catalyzes reversible hydration of cyanamide into urea. | ||
==Structure== | ==Structure== | ||
| Line 45: | Line 47: | ||
== Function == | == Function == | ||
| - | 1f2w | + | 1f2w catalyzes the hydration of cyanamide into urea according to the equation : |
[[Image:Cyanamide.png]] | [[Image:Cyanamide.png]] | ||
| - | Essential for bone resorption and osteoclast differentiation (By similarity). Reversible hydration of carbon dioxide. Can hydrate cyanamide to urea. Involved in the regulation of fluid secretion into the anterior chamber of the eye | ||
| - | [[Image:D7423ca6daa1149f361c10b977acad36.jpg]] | ||
==Mechanism of Action== | ==Mechanism of Action== | ||
| - | + | The 1f2w protein allows the cyanamide to bind the metal ion within the CA active site, which is adding to the coordination sphere. The cyanamid attacks the zinc ion (nucleophilic attack). Afertwards the water molecule performs a nucleophilic attack on the zinc-activated cyanamide substrate forming urea which remains bound to the metal for several hours. The urea molecule is directly coordinated to the active site Zn(II) ion through a protonated nitrogen atom. Several hydrogen bonds involving active site residues Thr199 and Thr200 as well as three water molecules (Wat99, Wat122, and Wat123) further stabilize the urea-hCA II adduct. Kinetic studies in solution further proved that urea acts as a tight binding inhibitor of the two isozymes hCA I and hCA II, with very slow binding kinetics (k(on) = 2.5 x 10(-5)s(-1)M(-1)). A mechanism to explain the hydration process of cyanamide by CAs, as well as the tight binding of urea in the active site, is also proposed based on the hypothesis that urea is deprotonated when bound to the enzyme. Cyanamide is thus the first true suicide substrate of this enzyme for which binding has been documented by means of X-ray crystallographic and spectroscopic studies. | |
The three-dimensional structure of a possible intermediate in the hydration reaction of cyanamide to urea catalyzed by human carbonic anhydrase II (hCAII) has been determined by cryocrystallographic techniques. The crystal structure shows that two different adducts are formed under the experimental conditions and that they have different occupancy in the crystal. The high occupancy form consists of a binary hCAII-cyanamide complex where the substrate has replaced the zinc-bound hydroxide anion present in the native enzyme, maintaining the tetrahedral geometry around the metal ion. The second, low-occupancy form consists of a hCAII-cyanamide-water ternary complex where the catalytic zinc ion, still being bound to cyanamide, is approached by a water molecule in a five-coordinate adduct. While the first form can be considered a nonproductive complex, the second form may represent an intermediate state of the catalyzed reaction where the water molecule is about to perform a nucleophilic attack on the zinc-activated cyanamide substrate. The structural evidence is consistent with the kinetic data previously reported about this recently described hydrolytic reaction catalyzed by hCAII, and indicates that a different mechanism with respect to that generally accepted for the physiologic carbon dioxide hydration reaction may be adopted by the enzyme, depending on the substrate chemical properties. | The three-dimensional structure of a possible intermediate in the hydration reaction of cyanamide to urea catalyzed by human carbonic anhydrase II (hCAII) has been determined by cryocrystallographic techniques. The crystal structure shows that two different adducts are formed under the experimental conditions and that they have different occupancy in the crystal. The high occupancy form consists of a binary hCAII-cyanamide complex where the substrate has replaced the zinc-bound hydroxide anion present in the native enzyme, maintaining the tetrahedral geometry around the metal ion. The second, low-occupancy form consists of a hCAII-cyanamide-water ternary complex where the catalytic zinc ion, still being bound to cyanamide, is approached by a water molecule in a five-coordinate adduct. While the first form can be considered a nonproductive complex, the second form may represent an intermediate state of the catalyzed reaction where the water molecule is about to perform a nucleophilic attack on the zinc-activated cyanamide substrate. The structural evidence is consistent with the kinetic data previously reported about this recently described hydrolytic reaction catalyzed by hCAII, and indicates that a different mechanism with respect to that generally accepted for the physiologic carbon dioxide hydration reaction may be adopted by the enzyme, depending on the substrate chemical properties. | ||
Revision as of 18:01, 8 January 2015
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
1f2w: Carbonic Anhydrase II
| |||||||||||