Sandbox Reserved 975
From Proteopedia
| Line 9: | Line 9: | ||
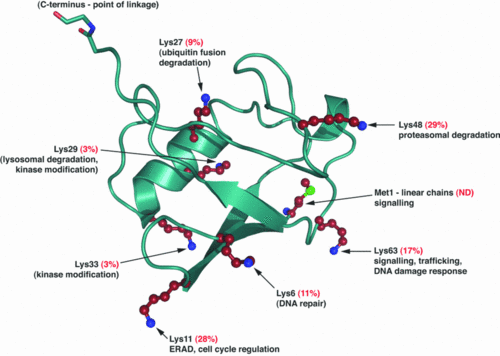
The Ubiquitin is a small protein of 76 aa. | The Ubiquitin is a small protein of 76 aa. | ||
| + | |||
| + | [[Image:Bst0370937a03.gif|500px]] | ||
Ubiquitination is a post translationnal modification where an ubiquitin is attached to a protein. This modification has several consequences, it can lead to the degradation of the protein via the proteasome, it can change the protein localization or it can alter the interaction of the protein with other factors, this is why this modification plays an important role in the control of many cellular processes. | Ubiquitination is a post translationnal modification where an ubiquitin is attached to a protein. This modification has several consequences, it can lead to the degradation of the protein via the proteasome, it can change the protein localization or it can alter the interaction of the protein with other factors, this is why this modification plays an important role in the control of many cellular processes. | ||
Current revision
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
SUMO-conjugating enzyme UBC9 EC:6.3.2.
| |||||||||||
References
- Allan D. Capili and Christopher D. Lima - Structure and analysis of a complex between SUMO and Ubc9 illustrates features of a conserved E2-Ubl interaction. Consulted the 28/12/2014 : http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1940065/
- Ubiquitin from wikipedia : http://en.wikipedia.org/wiki/Ubiquitin. Consulted the 27/12/2014
- Harry Tong, Guus Hateboer, Anastassis Perrakis, René Bernards and Titia K. Sixma - Crystal Structure of Murine/Human Ubc9 Provides Insight into the Variability of the Ubiquitin-conjugating System. Consulted the 28/12/2014 : http://www.jbc.org/content/272/34/21381.full
- Uniprot P63279- UBC9_HUMAN - SUMO-conjugating enzyme UBC9 : http://www.uniprot.org/uniprot/P63279. Consulted the 27/12/2014