This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 962
From Proteopedia
| Line 27: | Line 27: | ||
• The <scene name='60/604481/Segment_1/1'>first segment</scene> from the aminoacids 41 to 62 and from 165 to 292 : carrys the <scene name='60/604481/Helices_segment_1/1'>alpha helices</scene> A, F, H, E, I and G. The helices G, H and I are the <scene name='60/604481/Helices_c_terminal/1'>C-terminal helices</scene> from the aminoacid 249 to 284. This segment carrys also the <scene name='60/604481/Sheets_segment_1/1'>bêta-sheets</scene> from 5 to 11. | • The <scene name='60/604481/Segment_1/1'>first segment</scene> from the aminoacids 41 to 62 and from 165 to 292 : carrys the <scene name='60/604481/Helices_segment_1/1'>alpha helices</scene> A, F, H, E, I and G. The helices G, H and I are the <scene name='60/604481/Helices_c_terminal/1'>C-terminal helices</scene> from the aminoacid 249 to 284. This segment carrys also the <scene name='60/604481/Sheets_segment_1/1'>bêta-sheets</scene> from 5 to 11. | ||
| - | • The <scene name='60/604481/Segment_2/1'>second segment</scene> from the aminoacids 63 to 164 : carrys the alpha helices B, C, D and the bêta-sheets from 1 to 4. | + | • The <scene name='60/604481/Segment_2/1'>second segment</scene> from the aminoacids 63 to 164 : carrys the <scene name='60/604481/Helices_segment_2/1'>alpha helices</scene> B, C, D and the bêta-sheets from 1 to 4. |
Revision as of 20:52, 8 January 2015
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
mRNA Cap (Guanine-N7) Methyltransferase (Ecm1)
The mRNA Cap (Guanine-N7) Methyltransferase is an enzyme which catalyses the reaction of capping the C-terminal domain of a mRNA with a m7G(5')pppR cap.
|
Contents |
Biological role
In eukaryotic cells the synthezise of mRNA is followed by a process of maturation. The best-known modifications are the polyadenylation, the splicing and the (Guanine-N7) capping. The capping is an important step of the maturation. It consists in adding a 7-methylguanosine on the first nucleotide of the mRNA with a diphosphate bonding. The enzyme catalysing this reaction is the mRNA Cap (Guanine-N7) Methyltransferase.
The cap allows the mRNA to be recognized by a protein complex which is involved in traduction initiation.
Structure
This protein measures 292 aminoacids in lenght.
The structure of ECM1 (the smallest cap methyltranferase identified) is similar to the structure of the whole family of cap methyltransferase. We can indentify structural repeatings characteristic of the second class of the family : amongst others alpha helices or bêta-sheets can be found.
The structure of the protein could be divided in two segments :
• The from the aminoacids 41 to 62 and from 165 to 292 : carrys the A, F, H, E, I and G. The helices G, H and I are the from the aminoacid 249 to 284. This segment carrys also the from 5 to 11.
• The from the aminoacids 63 to 164 : carrys the B, C, D and the bêta-sheets from 1 to 4.
Interaction
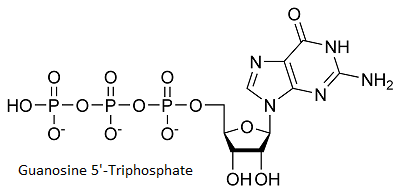
Cap Analog Binding (Guanosine 5'-Triphosphate)
binds to the enzyme in a pocket near AdoHcy. (Tyr 145, Leu216, Leu217, Asp218, Ser219, Tyr284) are involded in the binding of the cap analog, more precisely they interact with guanine and with the The cap makes Van der Walls contacts with side chains fromGTP makes a hydrogen bond with and a water mediated bond with . [1]
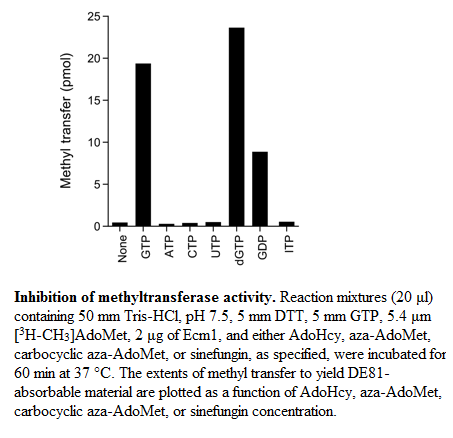
As we can see on the figure above[2] , the enzyme specifically binds to guanine. This specificity is achieved through different recognitions. The N-1 atom of adenine is unprotonated, this prevent the interaction of adenine with Ecm1. Ecm1 contact the of guanine and permit an additional discrimination between guanine and adenine. Moreover the fact that ITP is not a substrate for Ecm1 shows that the interactions between Ecm1 and are important for substrate binding.We also remark that the methyltransferase is not able to discrminate between and desoxyribose nucleoside sugars.
AdoHcy Binding (S-Adenosyl-L-Homocysteine)
The mRNA Cap Methyltransferase bind to which is the product of the methyl donor AdoMet after the methylation. The interaction describe here are applicable to AdoMet too. AdoHcys is in a pocket formed by amino acids of segment 2.(Lys54, Gly72, Asp78, Asp94, Ile95, Asp122, Ser124, Gln140, Phe141, Ser142) are involved in the stabilisation of AdoHcys.
The interactions between AdoHcys and the enzyme are made of : - Hydrogen bonds mediated by - Van der Walls interactions mediated by - An electrostatic interaction mediated by- A water mediated contact mediated by [1]
Mechanism
This enzyme catalyse N-methyl transfer from AdoMet (S-adenosylmethionine) to GpppRNA, this reaction produce 7-methyl-GpppRNA and AdoHcy. This reaction is made through a SN2 mechanism. We remark that there is no contact between the enzyme and or the AdoMet methyl carbon.Indeed the enzyme does not stabilize the transition state of the chemical reaction, does not promote the activation of the nucleophile or the expulsion of the leaving group. mRNA Cap Methyltransferase brings the two substrates closer and orientates the substrates to facilitate the methyl transfer. [1]
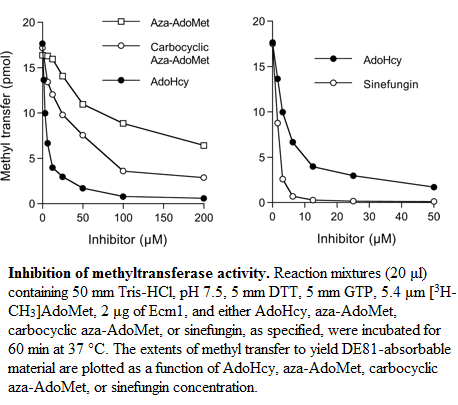
Inhibition
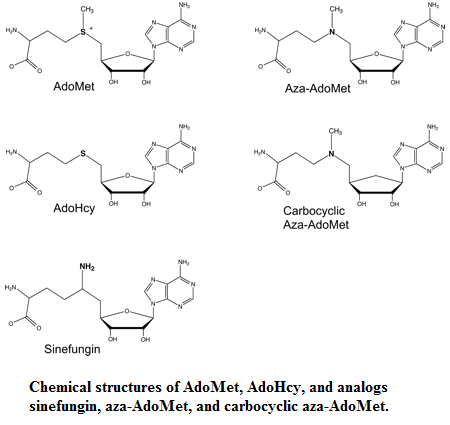
- AdoHcy
- Sinefugin
- Aza-AdoMet & carbocyclic aza-AdoMet
Aza-AdoMet and carbocyclic aza-AdoMet are analogs of AdoMet too. In these two molecules the sulfur atom is replaced by nitrogen. In the carbocyclic derivate the O4-atom of the ribose is replaced by a methylene group. These two molecules are weak inhibitors of Ecm1. The IC50 value of Aza-AdoMet is 100μm and of carbocyclic aza-AdoMet is 35μm [2]
Related Structure
1ri2 : Ecm1 associated with GTP only 1ri3 : Ecm1 associated with AdoHcy only 1ri4 : Ecm1 associated with AdoMet 1ri5 : Ecm1 only
References
- ↑ 1.0 1.1 1.2 Fabrega C, Hausmann S, Shen V, Shuman S, Lima CD. Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol Cell. 2004 Jan 16;13(1):77-89. PMID:14731396
- ↑ 2.0 2.1 2.2 2.3 Hausmann S, Zheng S, Fabrega C, Schneller SW, Lima CD, Shuman S. Encephalitozoon cuniculi mRNA cap (guanine N-7) methyltransferase: methyl acceptor specificity, inhibition BY S-adenosylmethionine analogs, and structure-guided mutational analysis. J Biol Chem. 2005 May 27;280(21):20404-12. Epub 2005 Mar 9. PMID:15760890 doi:10.1074/jbc.M501073200
Proteopedia page contributors and editors
Aline Girardet & Laure Hertzog