We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 951
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
==Biological context== | ==Biological context== | ||
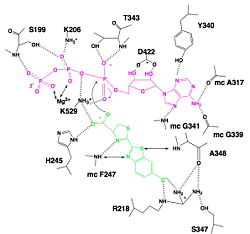
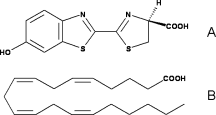
| - | Firefly, also named Photinus pyralis, is a bioluminescent insect. They are able to produce light by an energetic process, in order to attract its mate. The enzyme responsible of this light producing is luciferase, also known as luciferin-4-monooxygenase (EC: 1.13.12.7). It acts also as a ligase. This enzyme has various applications in the biotechnology field. In fact, it is used in chemical biology and drug trials. As there is no light production by mammals, luciferase is really a great tool for researchers. But this light emission depends on the environmental conditions. For example, they help to detect protein-protein interactions, to track cells in vivo in order to analysis the development of disease at the molecular level in real-time , to monitor the transcriptional and post-transcriptional regulation of specific gens, to control apoptosis, to label cancer cells, to detect environmental contaminations … | + | Firefly, also named Photinus pyralis, is a bioluminescent insect. They are able to produce light by an energetic process, in order to attract its mate. The enzyme responsible of this light producing is luciferase, also known as luciferin-4-monooxygenase (EC: 1.13.12.7). It acts also as a ligase. This enzyme has various applications in the biotechnology field. In fact, it is used in chemical biology and drug trials. As there is no light production by mammals, luciferase is really a great tool for researchers. But this light emission depends on the environmental conditions. For example, they help to detect protein-protein interactions, to track cells in vivo in order to analysis the development of disease at the molecular level in real-time , to monitor the transcriptional and post-transcriptional regulation of specific gens, to control apoptosis, to label cancer cells, to detect environmental contaminations … <ref>PMID: 15722018</ref> |
Revision as of 11:46, 9 January 2015
| |||||||||||
References
- ↑ Welsh DK, Kay SA. Bioluminescence imaging in living organisms. Curr Opin Biotechnol. 2005 Feb;16(1):73-8. PMID:15722018 doi:http://dx.doi.org/10.1016/j.copbio.2004.12.006
- ↑ Marques SM, Esteves da Silva JC. Firefly bioluminescence: a mechanistic approach of luciferase catalyzed reactions. IUBMB Life. 2009 Jan;61(1):6-17. PMID:18949818 doi:10.1002/iub.134
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Conti E, Franks NP, Brick P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure. 1996 Mar 15;4(3):287-98. PMID:8805533
- ↑ Photobiology
- ↑ Photobiology
- ↑ Photobiology
- ↑ Marques SM, Esteves da Silva JC. Firefly bioluminescence: a mechanistic approach of luciferase catalyzed reactions. IUBMB Life. 2009 Jan;61(1):6-17. PMID:18949818 doi:10.1002/iub.134
- ↑ Hosseinkhani S. Molecular enigma of multicolor bioluminescence of firefly luciferase. Cell Mol Life Sci. 2011 Apr;68(7):1167-82. doi: 10.1007/s00018-010-0607-0. Epub, 2010 Dec 28. PMID:21188462 doi:http://dx.doi.org/10.1007/s00018-010-0607-0
- ↑ Inouye S. Firefly luciferase: an adenylate-forming enzyme for multicatalytic functions. Cell Mol Life Sci. 2010 Feb;67(3):387-404. Epub 2009 Oct 27. PMID:19859663 doi:10.1007/s00018-009-0170-8