We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 951
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
Thus, this oxydo-reductase is involved in severals reactions. | Thus, this oxydo-reductase is involved in severals reactions. | ||
=====Light emission===== | =====Light emission===== | ||
| - | + | Luciferase can react to emit light. In this reaction, luciferase firstly synthetized luciferin-AMP from luciferin and ATP, using a Mg2+ ion to offset the negative charges of the phosphate groups. Then, luciferase turns the luciferin-AMP into oxyluciferin in an excited state thanks to a dioxygen. This step releases AMP and CO2. The excited oxyluciferin relaxes and looses a photon, so light is emitted. | |
| - | The wavelength of the light can vary with the pH : at the physiological pH, the emitted light is green and at a lower pH, the color is red.<ref name =''first''>PMID:8805533</ref> | + | The wavelength of the light can vary with the pH, which can be explained by the luciferase structure: at the physiological pH, the emitted light is green and at a lower pH, the color is red.<ref name =''first''>PMID:8805533</ref> |
=====Fatty-acyl-CoA synthesis===== | =====Fatty-acyl-CoA synthesis===== | ||
This reaction uses similar reaction but instead of taking luciferin as ligand, it takes fatty acids. Using ATP-Mg2+, it firstly forms fatty-acyl-AMP. And then, CoA-SH attacks the carboxylic group of fatty-acyl-AMP and so forms fatty-acyl-CoA. | This reaction uses similar reaction but instead of taking luciferin as ligand, it takes fatty acids. Using ATP-Mg2+, it firstly forms fatty-acyl-AMP. And then, CoA-SH attacks the carboxylic group of fatty-acyl-AMP and so forms fatty-acyl-CoA. | ||
| Line 29: | Line 29: | ||
== Structure related to functions == | == Structure related to functions == | ||
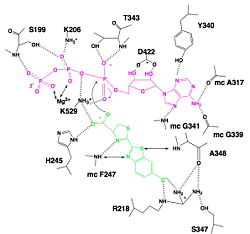
| - | The most known reaction of luciferase is the light emission where luciferase uses luciferin, ATP and O2 as substrates. | + | The most known reaction of luciferase is the light emission where luciferase uses luciferin, ATP and O2 as substrates. But there are some other reactions which can uses fatty acids and coenzyme A. So the active site of the luciferase can theorically bind all these compounds. |
====Interactions with ligands==== | ====Interactions with ligands==== | ||
| - | |||
The active site is not strictly highlighted according to the actual state of studies but some residues and motifs strongly modified have been determined and this conformation enables to find the active site. Many of these conserved residue are located on the core of the β-barrel and on the small C-terminal domain and in the surface of the N-terminal domain, which forms a <scene name='60/604470/Depression_c-ter_and_n-ter/1'>depression</scene>. However, this depression is too large to enable interactions between residues and substrates, so it is thought that a conformational change occurs and sandwiches the substrates, forming the active site. This conformational change provide a suitable environment for light production because of water molecules will be excluded from the active site, favouring intramolecular reactions. Residues also follow a <scene name='60/604470/Cleft/1'>cleft</scene> caused by of the <scene name='60/604470/Beta_sheet_b/3'>Beta-sheet B</scene> against the <scene name='60/604470/Beta_barrel/3'>Beta-barrel</scene>.<ref name =''fourth'>PMID:8805533</ref> | The active site is not strictly highlighted according to the actual state of studies but some residues and motifs strongly modified have been determined and this conformation enables to find the active site. Many of these conserved residue are located on the core of the β-barrel and on the small C-terminal domain and in the surface of the N-terminal domain, which forms a <scene name='60/604470/Depression_c-ter_and_n-ter/1'>depression</scene>. However, this depression is too large to enable interactions between residues and substrates, so it is thought that a conformational change occurs and sandwiches the substrates, forming the active site. This conformational change provide a suitable environment for light production because of water molecules will be excluded from the active site, favouring intramolecular reactions. Residues also follow a <scene name='60/604470/Cleft/1'>cleft</scene> caused by of the <scene name='60/604470/Beta_sheet_b/3'>Beta-sheet B</scene> against the <scene name='60/604470/Beta_barrel/3'>Beta-barrel</scene>.<ref name =''fourth'>PMID:8805533</ref> | ||
| Line 44: | Line 43: | ||
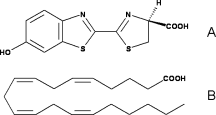
[[Image:Luciferin_fatty-acid.gif|250px|left|thumb|Comparison of the chemical structures of (A) firefly | [[Image:Luciferin_fatty-acid.gif|250px|left|thumb|Comparison of the chemical structures of (A) firefly | ||
D-LH2 and (B) arachidonic acid,<ref>PMID:18949818</ref>]] | D-LH2 and (B) arachidonic acid,<ref>PMID:18949818</ref>]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
====Color modulation==== | ====Color modulation==== | ||
| - | |||
When the pH is low, the color of light changes. This is probably due to the hydrogen bonds network between substrates, residues of the cleft and water. Indeed, this network triggers to an external electrostatic potential which stabilizes a charge created during the reaction of light emission. The lower pH leads to a weakening of the network, so the energy which can be emitted decreases and the wavelength of photon increases and becomes more red.<ref>PMID:21188462</ref> | When the pH is low, the color of light changes. This is probably due to the hydrogen bonds network between substrates, residues of the cleft and water. Indeed, this network triggers to an external electrostatic potential which stabilizes a charge created during the reaction of light emission. The lower pH leads to a weakening of the network, so the energy which can be emitted decreases and the wavelength of photon increases and becomes more red.<ref>PMID:21188462</ref> | ||
| - | == Evolution == | ||
| + | == Evolution == | ||
The number of proteins related to luciferase is growing exponentially. They are a lot of enzymes very different involved in a lot of mechanisms such as biosynthesis of siderophores, of antibiotics, fatty acid:coenzyme A ligase, and so on... | The number of proteins related to luciferase is growing exponentially. They are a lot of enzymes very different involved in a lot of mechanisms such as biosynthesis of siderophores, of antibiotics, fatty acid:coenzyme A ligase, and so on... | ||
All the coenzyme A ligase show a very high level of similarity : indeed, each of these enzyme related to luciferase catalyze the adenylation of a carboxylic acid substrate using ATP-Mg2+ and then, the ligation of the activated carboxylic acid with an acceptor. | All the coenzyme A ligase show a very high level of similarity : indeed, each of these enzyme related to luciferase catalyze the adenylation of a carboxylic acid substrate using ATP-Mg2+ and then, the ligation of the activated carboxylic acid with an acceptor. | ||
Revision as of 12:15, 9 January 2015
| |||||||||||
References
- ↑ Welsh DK, Kay SA. Bioluminescence imaging in living organisms. Curr Opin Biotechnol. 2005 Feb;16(1):73-8. PMID:15722018 doi:http://dx.doi.org/10.1016/j.copbio.2004.12.006
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Conti E, Franks NP, Brick P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure. 1996 Mar 15;4(3):287-98. PMID:8805533
- ↑ Marques SM, Esteves da Silva JC. Firefly bioluminescence: a mechanistic approach of luciferase catalyzed reactions. IUBMB Life. 2009 Jan;61(1):6-17. PMID:18949818 doi:10.1002/iub.134
- ↑ Photobiology
- ↑ Photobiology
- ↑ Photobiology
- ↑ Marques SM, Esteves da Silva JC. Firefly bioluminescence: a mechanistic approach of luciferase catalyzed reactions. IUBMB Life. 2009 Jan;61(1):6-17. PMID:18949818 doi:10.1002/iub.134
- ↑ Hosseinkhani S. Molecular enigma of multicolor bioluminescence of firefly luciferase. Cell Mol Life Sci. 2011 Apr;68(7):1167-82. doi: 10.1007/s00018-010-0607-0. Epub, 2010 Dec 28. PMID:21188462 doi:http://dx.doi.org/10.1007/s00018-010-0607-0
- ↑ Inouye S. Firefly luciferase: an adenylate-forming enzyme for multicatalytic functions. Cell Mol Life Sci. 2010 Feb;67(3):387-404. Epub 2009 Oct 27. PMID:19859663 doi:10.1007/s00018-009-0170-8