Biological context
Firefly, also named Photinus pyralis, is a bioluminescent insect. They are able to produce light by an energetic process, in order to attract its mate. The enzyme responsible of this light producing is luciferase, also known as luciferin-4-monooxygenase (EC: 1.13.12.7). It acts also as a ligase. This enzyme has various applications in the biotechnology field. In fact, it is used in chemical biology and drug trials. As there is no light production by mammals, luciferase is really a great tool for researchers. But this light emission depends on the environmental conditions. For example, they help to detect protein-protein interactions, to track cells in vivo in order to analysis the development of disease at the molecular level in real-time , to monitor the transcriptional and post-transcriptional regulation of specific gens, to control apoptosis, to label cancer cells, to detect environmental contaminations … [1]
Thus, this oxydo-reductase is involved in severals reactions.
Light emission
Luciferase can react to emit light. In this reaction, luciferase firstly synthetized luciferin-AMP from luciferin and ATP, using a Mg2+ ion to offset the negative charges of the phosphate groups. Then, luciferase turns the luciferin-AMP into oxyluciferin in an excited state thanks to a dioxygen. This step releases AMP and CO2. The excited oxyluciferin relaxes and looses a photon, so light is emitted.
The wavelength of the light can vary with the pH, which can be explained by the luciferase structure: at the physiological pH, the emitted light is green and at a lower pH, the color is red.[2]
Fatty-acyl-CoA synthesis
This reaction uses similar reaction but instead of taking luciferin as ligand, it takes fatty acids. Using ATP-Mg2+, it firstly forms fatty-acyl-AMP. And then, CoA-SH attacks the carboxylic group of fatty-acyl-AMP and so forms fatty-acyl-CoA.
Luciferin & Coenzyme A
It has been found that the reaction between luciferin and CoA is possible, forming in a first step luciferin-AMP and then luciferin-CoA. This reaction leads to an interesting biological phenomenon : when this reaction occurs in parallel of light emission reaction, we don't have a flash of light but a continuous light emission. This is because luciferin-AMP is a competitive inhibitor of the reaction whereas the luciferin-CoA is not. So the inhibition is deleted and the reaction continue to occur.[3]
Global Structure
Luciferase is a 62kDa protein and contains 550 amino acids. This enzyme can be divide into two domains. On the one hand, the major portion, corresponding to the . On the other hand, the small portion, corresponding to the .Those two domains are separated by a large cleft.[2]
The C-terminal domain
The sequence of amino acids of C-terminal domain is composed of contiguous residues and forms a type of lid upon the N-terminal domain. It contains two β sheets: the is composed of two short antiparallel strands and the is composed of 3 antiparallel strands, mixed with . Those helices are put toward the outside. It is α + β structure.[2]
The N-terminal domain
The sequence of amino acids of C-terminal domain is composed of non-contiguous residues. It falls into three subdomains (,, and ). The subdomains A and B corresponds to two β sheets, which are framed by . Each of the two β sheets subdomains are composed of 8 β strands and 6 helices. The β sheet A has . The β sheet B has . Those β sheets create a groove, closed on one end by the subdomain C, which is an antiparallel . [2]
Structure related to functions
The most known reaction of luciferase is the light emission where luciferase uses luciferin, ATP and O2 as substrates. But there are some other reactions which can use fatty acids and coenzyme A. So the active site of the luciferase can theorically bind all these compounds.
Interactions with ligands
The active site is not strictly highlighted according to the actual state of studies but some residues and motifs strongly modified have been determined and this conformation enables to find the active site. Many of these conserved residues are located on the core of the β-barrel, on the small C-terminal domain and in the surface of the N-terminal domain, which forms a . However, this depression is too large to enable interactions between residues and substrates, so it is thought that a conformational change occurs and sandwiches the substrates, forming the active site. This conformational change provides a suitable environment for light production because of exclusion of water molecules from the active site, favouring intramolecular reactions. Residues also follow a caused by of the against the .[2]
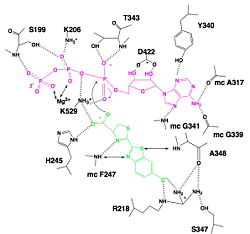
Hydrogen bonding between Luciferase and substrates luciferin (green), ATP (violet) and Mg2+,
[4] Interaction with ATP
We find a signal motif in luciferase which is where some residues like lysine are always conserved. This pattern enables ATP binding thanks to hydrogen bonds between residues and phosphates of ATP. There is another pattern : which takes a particular conformation because of hydrogen bonds between residues and maintain the adenosin ring of ATP.[2], [5]
Interaction with luciferin
Luciferase holds the luciferin with the specific residues , still with hydrogen bounds. Those bindings make the carboxylate oxygen of luciferin points toward the α phosphate of ATP, so the oxygen is well-positionned to attack the α phosphate. This promotes the luciferin-AMP formation.[2], [6]
Interaction with fatty acids
Fatty acids are highly similar to luciferin. Therefore, luciferase can use the luciferin binding site to bind fatty acids. That is why they can be used as substrates by luciferase and then, very high similar reaction as for luciferin occurs.
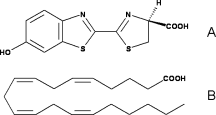
Comparison of the chemical structures of (A) firefly D-LH2 and (B) arachidonic acid,
[7] Color modulation
When the pH is low, the color of light changes. This is probably due to the hydrogen bonds network between substrates, residues of the cleft and water. Indeed, this network triggers to an external electrostatic potential which stabilizes a charge created during the reaction of light emission. The lower pH leads to a weakening of the network, so the energy which can be emitted decreases and the wavelength of photon increases and becomes more red.[8]
Evolution
The number of proteins related to luciferase is growing exponentially. They are a lot of different enzymes involved in a lot of mechanisms such as biosynthesis of siderophores, of antibiotics, of fatty acid :coenzyme A ligase, and so on...
All the coenzyme A ligase show a very high level of similarity : indeed, each of these enzymes related to luciferase catalyze the adenylation of a carboxylic acid substrate using ATP-Mg2+ and then, the ligation of the activated carboxylic acid with an acceptor.
That is why luciferase is more and more considered as coming from a common ancestor involved in this kind of reaction and the light production would only be a side effect of the reaction.[9]


