We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Editing User:Pauline Hanns/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
Cytokine deshydrogenase are '''extracellular''' and '''monomeric''' proteins with a molecular weight of 63kDa.<ref name="Kopečnýa">PMID: 20478354 </ref><ref name="Kopečnýa2">PMID: 18571199 </ref> For protein production purposes, ZmCKO1 precursor protein was truncated by deletion of 18 N-terminal amino acids to produce the expected mature enzyme. <ref name="Kopečnýa3">PMID: 15927342 </ref> | Cytokine deshydrogenase are '''extracellular''' and '''monomeric''' proteins with a molecular weight of 63kDa.<ref name="Kopečnýa">PMID: 20478354 </ref><ref name="Kopečnýa2">PMID: 18571199 </ref> For protein production purposes, ZmCKO1 precursor protein was truncated by deletion of 18 N-terminal amino acids to produce the expected mature enzyme. <ref name="Kopečnýa3">PMID: 15927342 </ref> | ||
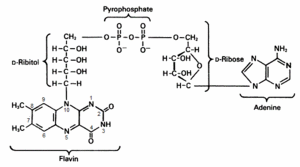
The Enzyme Classification number of CKX is EC [http://www.brenda-enzymes.info/php/result_flat.php4? 1.5.99.12] and this indicates that the enzyme is an '''oxydo reductase''' which acts on the CH-NH group of the donor. Consequently the reaction of CKX with its cytokinin substrate is a '''transfer of two electrons''' from the cytokinin to an '''electron acceptor''' which is in the case of CKX the '''F'''lavin '''A'''denine '''N'''ucleotide (FAD) cofactor. <ref name="Kopečnýa">PMID: 20478354 </ref> | The Enzyme Classification number of CKX is EC [http://www.brenda-enzymes.info/php/result_flat.php4? 1.5.99.12] and this indicates that the enzyme is an '''oxydo reductase''' which acts on the CH-NH group of the donor. Consequently the reaction of CKX with its cytokinin substrate is a '''transfer of two electrons''' from the cytokinin to an '''electron acceptor''' which is in the case of CKX the '''F'''lavin '''A'''denine '''N'''ucleotide (FAD) cofactor. <ref name="Kopečnýa">PMID: 20478354 </ref> | ||
| - | [[Image:FADESBS.gif|right|300px|thumb|'''F'''lavin '''A'''denine '''D'''inucleotide]] | + | [[Image:FADESBS.gif|right|300px|thumb|<center>'''F'''lavin '''A'''denine '''D'''inucleotide</center>]] |
In some papers the denomination '''CKO''' can be found for cytokinin deshydrogenase. Indeed in the VAO flavoprotein family most of the enzyme use '''molecular oxygen''' as electron acceptor to '''reoxidize''' the FAD cofactor. That’s why the enzyme was first called '''Cytokinin Oxidase''' (CKO). CKX is an exception in the family since the enzyme uses '''other compounds''' ,such as quinone, for electron acceptor and poorly reacts with oxygen. Consequently the enzyme is now called '''CKX''' and enters the category of '''dehydrogenase'''. <ref name="Frebortova">PMID: 19912568 </ref> <ref name="Malitoa">PMID: 15321719 </ref> | In some papers the denomination '''CKO''' can be found for cytokinin deshydrogenase. Indeed in the VAO flavoprotein family most of the enzyme use '''molecular oxygen''' as electron acceptor to '''reoxidize''' the FAD cofactor. That’s why the enzyme was first called '''Cytokinin Oxidase''' (CKO). CKX is an exception in the family since the enzyme uses '''other compounds''' ,such as quinone, for electron acceptor and poorly reacts with oxygen. Consequently the enzyme is now called '''CKX''' and enters the category of '''dehydrogenase'''. <ref name="Frebortova">PMID: 19912568 </ref> <ref name="Malitoa">PMID: 15321719 </ref> | ||
| Line 54: | Line 54: | ||
=='''Mechanism'''== | =='''Mechanism'''== | ||
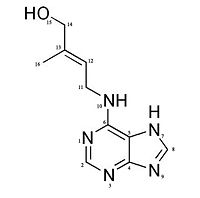
| - | [[Image:Reac2QKNESBS.jpg|center|450px|thumb|Mechanism of the reaction of the FAD cofactor on the substrate <ref name="Kopečnýa">PMID: 20478354 </ref>]] | + | [[Image:Reac2QKNESBS.jpg|center|450px|thumb|<center>Mechanism of the reaction of the FAD cofactor on the substrate <ref name="Kopečnýa">PMID: 20478354 </ref></center>]] |
The positioning of the C11 close to the N5 of the flavin suggests that electrons and protons are exchanged through a '''direct transfer from the substrate to the flavin'''. This implies a '''short-lived radical intermediate''' or the direct tranfer of a '''hydride anion'''.<ref name="Malitoa">PMID: 15321719 </ref> The attack of the N5 to the C11 create a '''carbocation''', on the cytokinin, which is stabilized by the N10–Asp 169 Hydrogen-bond interaction and the resonance effect in the oxidised imine product. | The positioning of the C11 close to the N5 of the flavin suggests that electrons and protons are exchanged through a '''direct transfer from the substrate to the flavin'''. This implies a '''short-lived radical intermediate''' or the direct tranfer of a '''hydride anion'''.<ref name="Malitoa">PMID: 15321719 </ref> The attack of the N5 to the C11 create a '''carbocation''', on the cytokinin, which is stabilized by the N10–Asp 169 Hydrogen-bond interaction and the resonance effect in the oxidised imine product. | ||
The reduced enzyme-product complex (FADH2) is '''reoxidised''' by using an '''organic electron acceptor''', the hydroxamic acid 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-on (DIMBOA) or more precisely, the free radicals generated by the reaction of laccase and peroxydase on the DIMBOA. The reoxidation of FADH2 allows the product (adenine and 3 methyl 2 butanal) release.<ref name="Malitoa">PMID: 15321719 </ref> | The reduced enzyme-product complex (FADH2) is '''reoxidised''' by using an '''organic electron acceptor''', the hydroxamic acid 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-on (DIMBOA) or more precisely, the free radicals generated by the reaction of laccase and peroxydase on the DIMBOA. The reoxidation of FADH2 allows the product (adenine and 3 methyl 2 butanal) release.<ref name="Malitoa">PMID: 15321719 </ref> | ||
Revision as of 16:49, 9 January 2015
2qkn
Crystal structure of Maize cytokinin oxidase/dehydrogenase complexed with phenylurea inhibitor CPPU
| |||||||||||