We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 972
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='3cww' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='3cww' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | + | Insulin Degrading Enzyme (or IDE) plays an important role in the cellular degradation of many molecules. Its activity was first related only to insulin, but now, we know that IDE interacts with insulin, glugacon and bradykinin. | |
| - | + | The IDE-bradykinin complex has a complexity in the substrate binding and recognition mechanism of IDE. | |
| - | + | ==Insuline degrading enzyme== | |
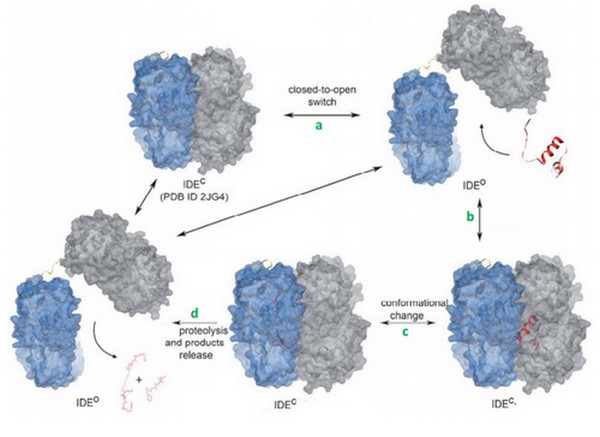
| - | + | <scene name='60/604491/Ide/1'>IDE</scene> (EC 3.4.24.56) is a human enzyme of the metallopeptidase family. It is composed by more than 1000 residues and has a huge catalytic cavity. It is made of 2 parts linked by a loop, and it switches between an open and a close state. The size of its catalytic chamber allows the binding of peptides (70 amino acids long). IDE hydrolyzes a lot of substrates which have many differents biological activities. Its substrate can be insuline, glucagon, amyline or bradykinin. This enzyme hydrolyses its substrates by cleaving them at different points. | |
| - | + | ||
| - | ==Insuline degrading enzyme | + | |
| - | <scene name='60/604491/Ide/1'>IDE</scene> (EC 3.4.24.56) is a human enzyme of the metallopeptidase family | + | |
| - | + | ||
| - | This enzyme hydrolyses its substrates by cleaving them at different points | + | |
===Exosite: an essential element for the catalysis=== | ===Exosite: an essential element for the catalysis=== | ||
| - | This is an essential component for the substrate hydrolysis. | + | Actually, previous studies reveal that IDE uses an exosite to interact with the N-terminal end of its substrate<ref>doi: 10.1074/jbc.M701590200</ref>. This exosite is completely different from the catalytic site, but it is an essential component for the substrate hydrolysis. Actually, it plays a role in the docking of substrates on IDE. Actually, when substrates arrive, they first bind to the exosite and then, they are translocated to the catalytic site. They have to change their conformation<ref> doi: 10.1038/nature05143</ref> in order to access to the catalytic site. It was also observed that the binding of bradykinin occurs at the exosite and not the catalytic site. |
===Catalytic mechanism=== | ===Catalytic mechanism=== | ||
| - | IDE can switch between its open and its close state. In the close state, IDE can not bind a substrate but it can hydrolyze it if it was already binding. In the other hand, in the open state, IDE can bind a substrate but it is less active because the catalytic site is not completly close. | + | [IDE] can switch between its open and its close state. In the close state, IDE can not bind a substrate but it can hydrolyze it if it was already binding. In the other hand, in the open state, IDE can bind a substrate but it is less active because the catalytic site is not completly close. |
The catalytic mechanism occurs in 4 steps: | The catalytic mechanism occurs in 4 steps: | ||
| Line 45: | Line 40: | ||
==Hypothetical role of bradykinin on IDE== | ==Hypothetical role of bradykinin on IDE== | ||
| - | Today, we can supposed that binding of bradykinin at the exosite stimulated the conformationnal change of IDE | + | Today, we can supposed that binding of bradykinin at the exosite stimulated the conformationnal change of IDE, from its open to its close state. |
We can also suggests that IDE binds <scene name='60/604491/Bradykinin/1'>2 bradykinins</scene> thanks to their small lenght. | We can also suggests that IDE binds <scene name='60/604491/Bradykinin/1'>2 bradykinins</scene> thanks to their small lenght. | ||
Binding of bradykinin or other short peptides to the exosite could play a regulatory role in substrate binding and cleavage by IDE<ref>doi: 10.1021/bi801192h</ref>. | Binding of bradykinin or other short peptides to the exosite could play a regulatory role in substrate binding and cleavage by IDE<ref>doi: 10.1021/bi801192h</ref>. | ||
Revision as of 22:11, 9 January 2015
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Im H, Manolopoulou M, Malito E, Shen Y, Zhao J, Neant-Fery M, Sun CY, Meredith SC, Sisodia SS, Leissring MA, Tang WJ. Structure of substrate-free human insulin-degrading enzyme (IDE) and biophysical analysis of ATP-induced conformational switch of IDE. J Biol Chem. 2007 Aug 31;282(35):25453-63. Epub 2007 Jul 5. PMID:17613531 doi:10.1074/jbc.M701590200
- ↑ Shen Y, Joachimiak A, Rosner MR, Tang WJ. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature. 2006 Oct 19;443(7113):870-4. Epub 2006 Oct 11. PMID:17051221 doi:10.1038/nature05143
- ↑ Song ES, Juliano MA, Juliano L, Hersh LB. Substrate activation of insulin-degrading enzyme (insulysin). A potential target for drug development. J Biol Chem. 2003 Dec 12;278(50):49789-94. Epub 2003 Oct 2. PMID:14527953 doi:http://dx.doi.org/10.1074/jbc.M308983200
- ↑ Im H, Manolopoulou M, Malito E, Shen Y, Zhao J, Neant-Fery M, Sun CY, Meredith SC, Sisodia SS, Leissring MA, Tang WJ. Structure of substrate-free human insulin-degrading enzyme (IDE) and biophysical analysis of ATP-induced conformational switch of IDE. J Biol Chem. 2007 Aug 31;282(35):25453-63. Epub 2007 Jul 5. PMID:17613531 doi:10.1074/jbc.M701590200
- ↑ Malito E, Ralat LA, Manolopoulou M, Tsay JL, Wadlington NL, Tang WJ. Molecular Bases for the Recognition of Short Peptide Substrates and Cysteine-Directed Modifications of Human Insulin-Degrading Enzyme. Biochemistry. 2008 Nov 6. PMID:18986166 doi:10.1021/bi801192h