Mycobacterium tuberculosis ArfA Rv0899
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
==Structure Section== | ==Structure Section== | ||
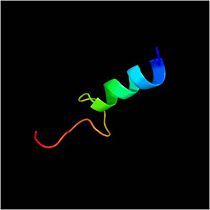
| - | The 326-residue protein contains three domains: an N-terminal domain (residues 1-72) that includes a sequence of 20 hydrophobic amino acids required for membrane translocation | + | The 326-residue protein contains three domains: an N-terminal domain (residues 1-72) that includes a sequence of 20 hydrophobic amino acids required for membrane translocation. |
| - | [[Image:N-ter domain.jpg| | + | |
| + | [[Image:N-ter domain.jpg|210px]] | ||
A central B domain ( <scene name='61/612805/Bon_domains/1'>residues 73-200</scene> ) with homology to the conserved putative lipid-binding BON (bacterial OsmY and nodulation) superfamily[http://www.ebi.ac.uk/interpro/entry/IPR014004] ,<scene name='61/612805/with conserved_g95_and_g164_in_bon/1'>with conserved G95 and G164 in BON superfamily</scene>, and a C domain (residues 201-326) with homology to the OmpA-C-like superfamily of periplasmic peptidoglycan-binding sequences, found in several types of bacterial membrane proteins. Residues 73-326 form a mixed alpha/beta-globular structure, encompassing two independently folded modules corresponding to the B and C domains connected by a flexible linker. The B domain folds with <scene name='61/612805/Sheet_and_helix/1'> three parallel/antiparallel alpha-helices packed against six parallel/antiparallel beta-strands that form a flat beta-sheet</scene> . .<scene name='61/612805/Surface/1'>The core is hydrophobic, while the exterior is polar and predominantly acidic</scene> | A central B domain ( <scene name='61/612805/Bon_domains/1'>residues 73-200</scene> ) with homology to the conserved putative lipid-binding BON (bacterial OsmY and nodulation) superfamily[http://www.ebi.ac.uk/interpro/entry/IPR014004] ,<scene name='61/612805/with conserved_g95_and_g164_in_bon/1'>with conserved G95 and G164 in BON superfamily</scene>, and a C domain (residues 201-326) with homology to the OmpA-C-like superfamily of periplasmic peptidoglycan-binding sequences, found in several types of bacterial membrane proteins. Residues 73-326 form a mixed alpha/beta-globular structure, encompassing two independently folded modules corresponding to the B and C domains connected by a flexible linker. The B domain folds with <scene name='61/612805/Sheet_and_helix/1'> three parallel/antiparallel alpha-helices packed against six parallel/antiparallel beta-strands that form a flat beta-sheet</scene> . .<scene name='61/612805/Surface/1'>The core is hydrophobic, while the exterior is polar and predominantly acidic</scene> | ||
Revision as of 19:43, 13 January 2015
| |||||||||||