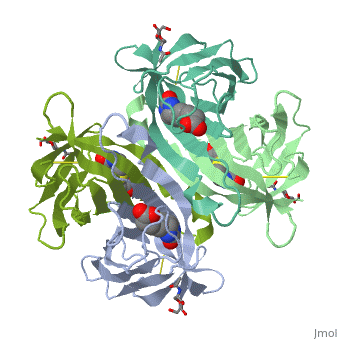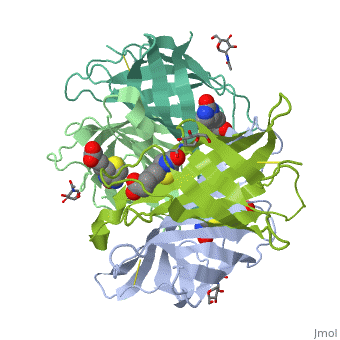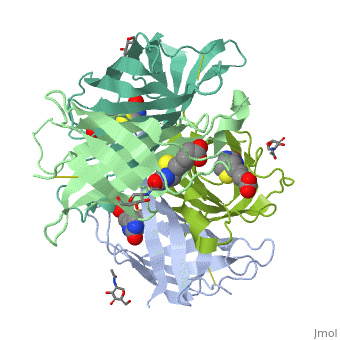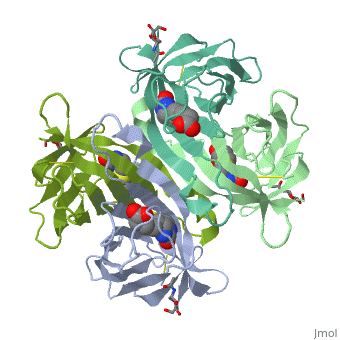User:Noam Gonen/Avidin
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds.[2] | Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds.[2] | ||
<StructureSection load='2avi' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='2avi' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
Revision as of 10:36, 17 January 2015
INTRODUCTION
Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds.[2]
| |||||||||||