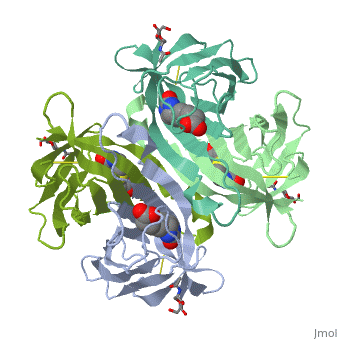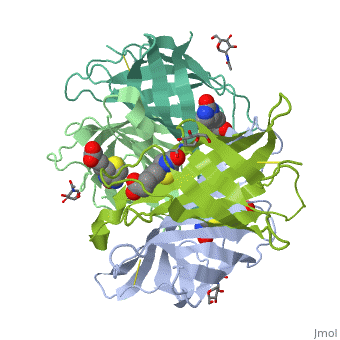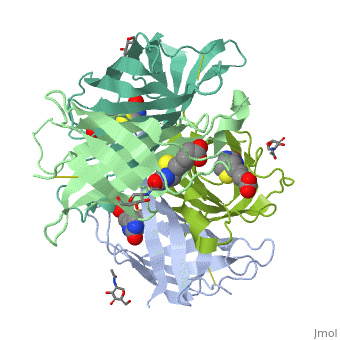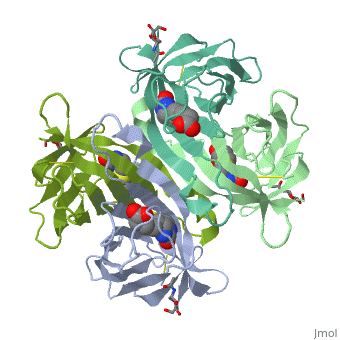User:Noam Gonen/Avidin
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds[2].The overall fold of the avidin monomer is constructed of eight antiparallel β-strands which form classical β-barrel. | The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds[2].The overall fold of the avidin monomer is constructed of eight antiparallel β-strands which form classical β-barrel. | ||
== Monomer- monomer interaction == | == Monomer- monomer interaction == | ||
| - | + | '''Interaction 1-2''' | |
| - | + | ||
| - | + | ||
Monomers are linked by hydrogen bond interactions between the respective N-terminal portions of β8-strands of each monomer. The β8-strands form a short antiparallel β-sheet. Each monomer contributes Trp-110 to its partner as an additional and very significant component of the biotin-binding site. When biotin is bound, interaction 1-2 is enhanced greatly, owing to the Trp-110-biotin interaction. The buried surface area of interaction is〖729Å〗^2. | Monomers are linked by hydrogen bond interactions between the respective N-terminal portions of β8-strands of each monomer. The β8-strands form a short antiparallel β-sheet. Each monomer contributes Trp-110 to its partner as an additional and very significant component of the biotin-binding site. When biotin is bound, interaction 1-2 is enhanced greatly, owing to the Trp-110-biotin interaction. The buried surface area of interaction is〖729Å〗^2. | ||
'''Interaction 1-3''' | '''Interaction 1-3''' | ||
Monomers interaction is relatively weak, involving only three equivalent hydrophobic residues from each monomer, Met-96, Val-115, and Ile-11. Resultant van der Waals interactions have the least contribution to the overall stability of the tetrameric structure of avidin. The buried surface area of interaction is〖120Å〗^2 . | Monomers interaction is relatively weak, involving only three equivalent hydrophobic residues from each monomer, Met-96, Val-115, and Ile-11. Resultant van der Waals interactions have the least contribution to the overall stability of the tetrameric structure of avidin. The buried surface area of interaction is〖120Å〗^2 . | ||
| + | |||
'''Interaction 1-4''' | '''Interaction 1-4''' | ||
Intricate interaction, which is decisive to the observed structural stability of the avidin tetramer.The cohesion of the two monomers, is so intimate that it is difficult to distinguish between them in the resultant dimer. Sections of four β -strands (β4, β5, β6, and β7) from each monomer take part in this extensive interaction.The buried surface area of interaction is〖1951Å〗^2 . | Intricate interaction, which is decisive to the observed structural stability of the avidin tetramer.The cohesion of the two monomers, is so intimate that it is difficult to distinguish between them in the resultant dimer. Sections of four β -strands (β4, β5, β6, and β7) from each monomer take part in this extensive interaction.The buried surface area of interaction is〖1951Å〗^2 . | ||
Revision as of 15:55, 17 January 2015
INTRODUCTION
Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs.
| |||||||||||