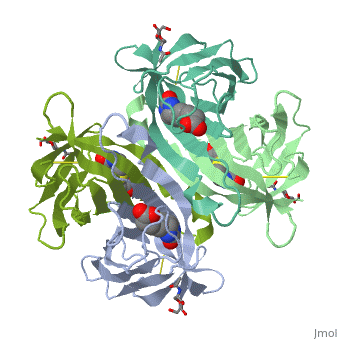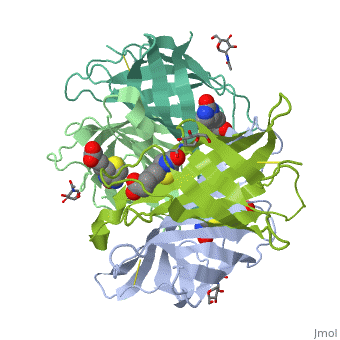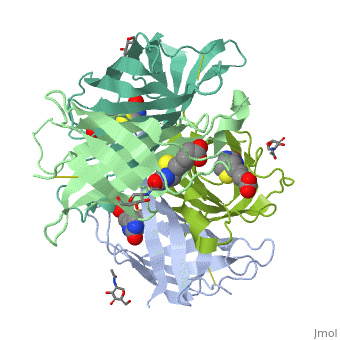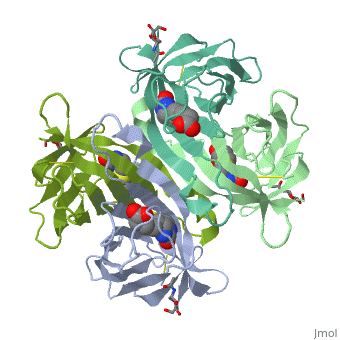User:Noam Gonen/Avidin
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structure == | == Structure == | ||
| - | The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ | + | The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ <math>10^-15</math> M, making it one of the strongest known non-covalent bonds[2].The overall fold of the avidin monomer is constructed of eight antiparallel β-strands which form classical β-barrel. |
== Monomer- monomer interaction == | == Monomer- monomer interaction == | ||
Revision as of 15:57, 17 January 2015
INTRODUCTION
Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs.
| |||||||||||