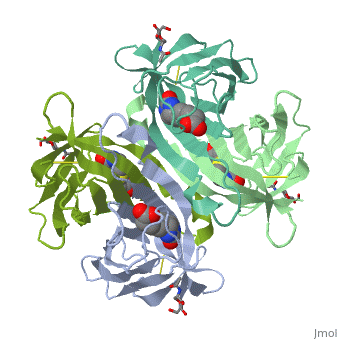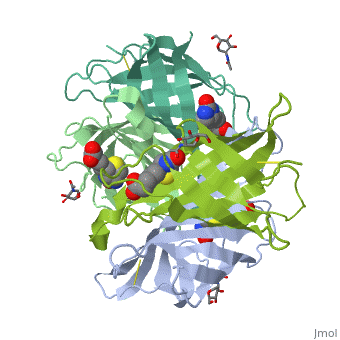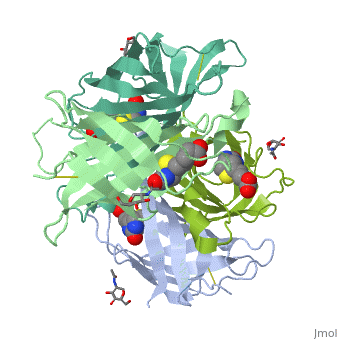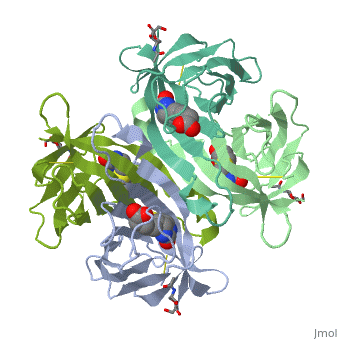User:Noam Gonen/Avidin
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structure == | == Structure == | ||
| - | The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ | + | The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ 10−15M, making it one of the strongest known non-covalent bonds[2].The overall fold of the avidin monomer is constructed of eight antiparallel β-strands which form classical β-barrel. |
== Monomer- monomer interaction == | == Monomer- monomer interaction == | ||
| Line 18: | Line 18: | ||
'''Interaction 1-4''' | '''Interaction 1-4''' | ||
Intricate interaction, which is decisive to the observed structural stability of the avidin tetramer.The cohesion of the two monomers, is so intimate that it is difficult to distinguish between them in the resultant dimer. Sections of four β -strands (β4, β5, β6, and β7) from each monomer take part in this extensive interaction.The buried surface area of interaction is〖1951Å〗^2 . | Intricate interaction, which is decisive to the observed structural stability of the avidin tetramer.The cohesion of the two monomers, is so intimate that it is difficult to distinguish between them in the resultant dimer. Sections of four β -strands (β4, β5, β6, and β7) from each monomer take part in this extensive interaction.The buried surface area of interaction is〖1951Å〗^2 . | ||
| - | == | + | ==Biotin binding == |
== Structural highlights == | == Structural highlights == | ||
Revision as of 16:00, 17 January 2015
INTRODUCTION
Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs.
| |||||||||||