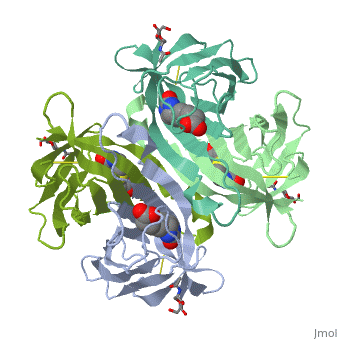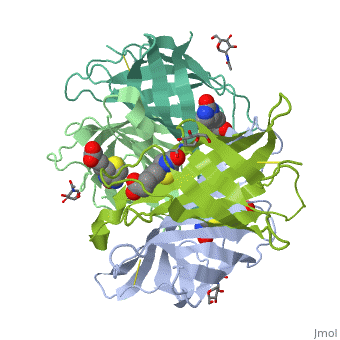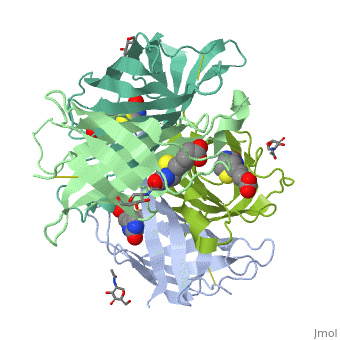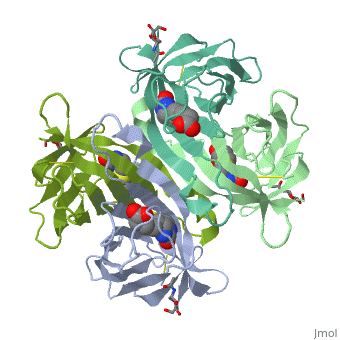User:Noam Gonen/Avidin
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
'''Hydrophobic residues in the binding site of avidin''' | '''Hydrophobic residues in the binding site of avidin''' | ||
| - | These include Trp-70, Phe-72, Phe-79, and Trp-97 from one monomer, and Trp-110 (dashed lines), which is provided by the adjacent symmetry-related monomer. | + | These include Trp-70, Phe-72, Phe-79, and Trp-97 from one monomer, and Trp-110 (dashed lines), which is provided by the adjacent symmetry-related monomer. |
| - | + | '''Hydrophilic interactions in the binding site of avidin''' | |
| + | |||
| + | Ureido oxygen of the biotin molecule forms three hydrogen bonds with the side chains of Asn-12, Ser-16, and Tyr-33 of avidin forming a tetrahedral oxyanion. In addition, each of the two ureido nitrogens participates in a single hydrogen-bond interaction with Thr-35 and Asn-118. The biotin sulfur may interact with Thr-77. | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 16:05, 17 January 2015
INTRODUCTION
Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs.
| |||||||||||