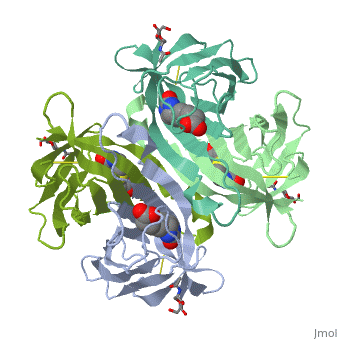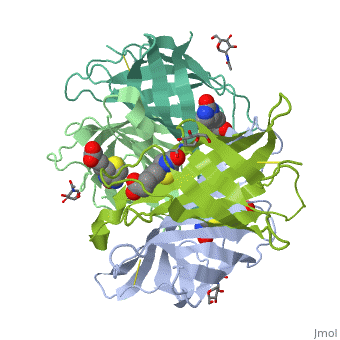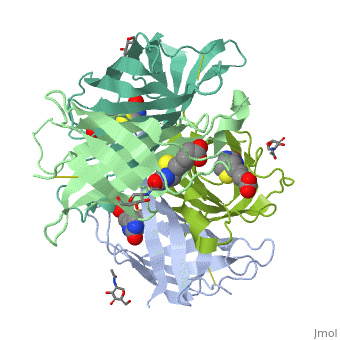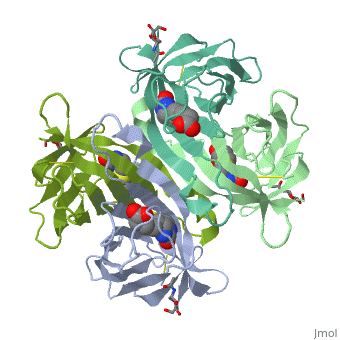User:Noam Gonen/Avidin
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
'''Interaction 1-2''' | '''Interaction 1-2''' | ||
| - | + | Monomers are linked by hydrogen bond interactions between the respective N-terminal portions of β8-strands of each monomer. The β8-strands form a short antiparallel β-sheet. Each monomer contributes Trp-110 to its partner as an additional and very significant component of the biotin-binding site. When biotin is bound, interaction 1-2 is enhanced greatly, owing to the Trp-110-biotin interaction. The buried surface area of interaction is〖729Å〗^2. | |
| - | Monomers are linked by hydrogen bond interactions between the respective N-terminal portions of β8-strands of each monomer. The β8-strands form a short antiparallel β-sheet. Each monomer contributes Trp-110 to its partner as an additional and very significant component of the biotin-binding site. When biotin is bound, interaction 1-2 is enhanced greatly, owing to the Trp-110-biotin interaction. The buried surface area of interaction is〖729Å〗^2. | + | |
'''Interaction 1-3''' | '''Interaction 1-3''' | ||
Revision as of 16:06, 17 January 2015
INTRODUCTION
Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs.
| |||||||||||