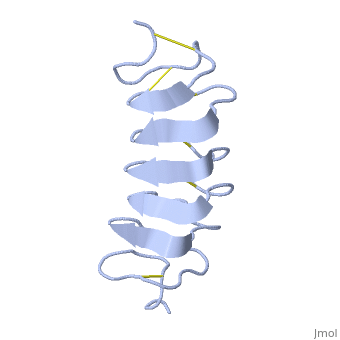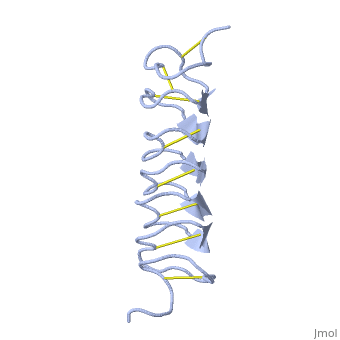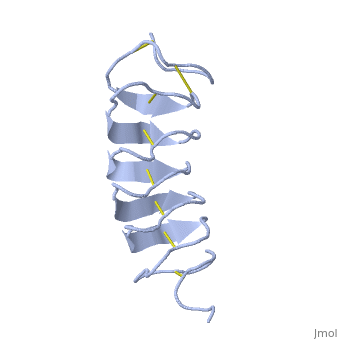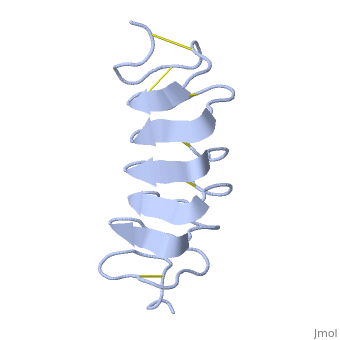
Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
== Function == | == Function == | ||
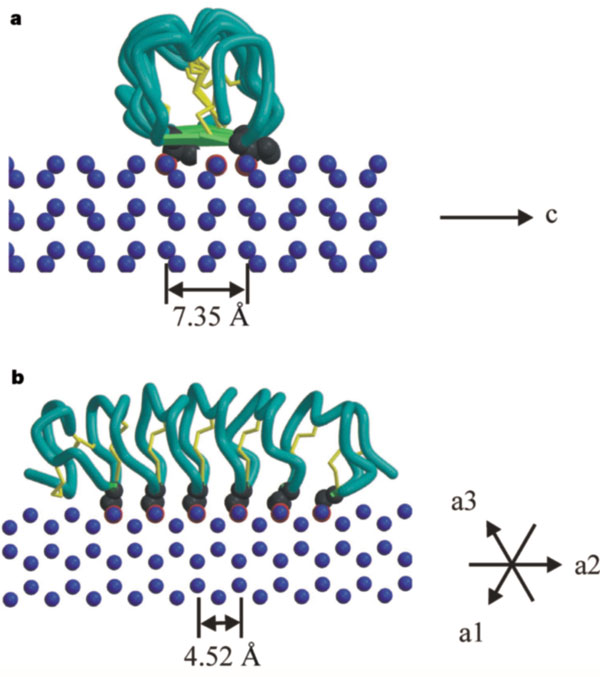
| - | The function of the ''Tm''AFP (And other Antifreeze protein) is Thermal Hysteresis(TH) .TH activity is occurs due to an adsorption inhibition mechanism that’s states that AFPs binds to ice surface and allow ice crystal growths only in surface regions between the bound AFP. Growing curvature causes an increase in surface energy, making the transformation of water into ice lass energetically favorable. Because of that the AFPs lower freezing temperature below the melting point. The difference between the melting and freezing point of the ice called thermal hysteresis activity.Due to the special structure of the protein, the TH activity can reach | + | The function of the ''Tm''AFP (And other Antifreeze protein) is Thermal Hysteresis(TH) .TH activity is occurs due to an adsorption inhibition mechanism that’s states that AFPs binds to ice surface and allow ice crystal growths only in surface regions between the bound AFP. Growing curvature causes an increase in surface energy, making the transformation of water into ice lass energetically favorable. Because of that the AFPs lower freezing temperature below the melting point. The difference between the melting and freezing point of the ice called thermal hysteresis activity.Due to the special structure of the protein, the TH activity can reach 6 celsius degree(Hyperactive protein).The maximum TH activity of moderate AFP is 1.5 celsius degree. |
Revision as of 10:21, 18 January 2015
| |||||||||||
References
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ Mitochondria as we don't know them. Tielens, Aloysius G.M et al. Trends in Biochemical Sciences , Volume 27 , Issue 11 , 564 - 572 doi:10.1016/S0968-0004(02)02193-X
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604