Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
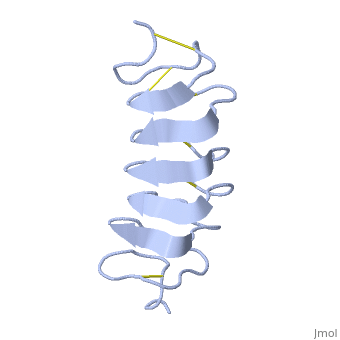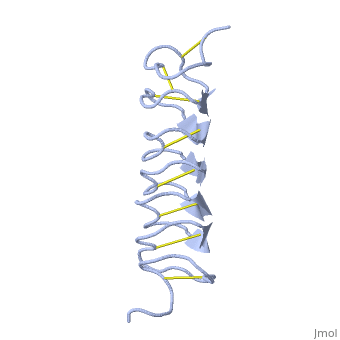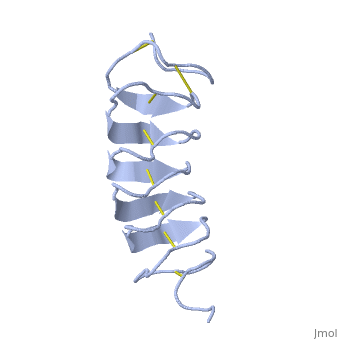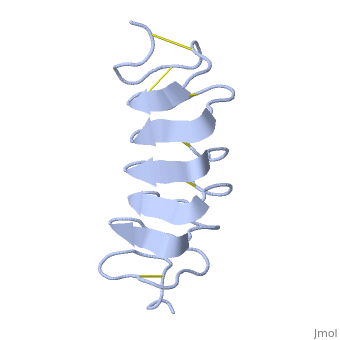
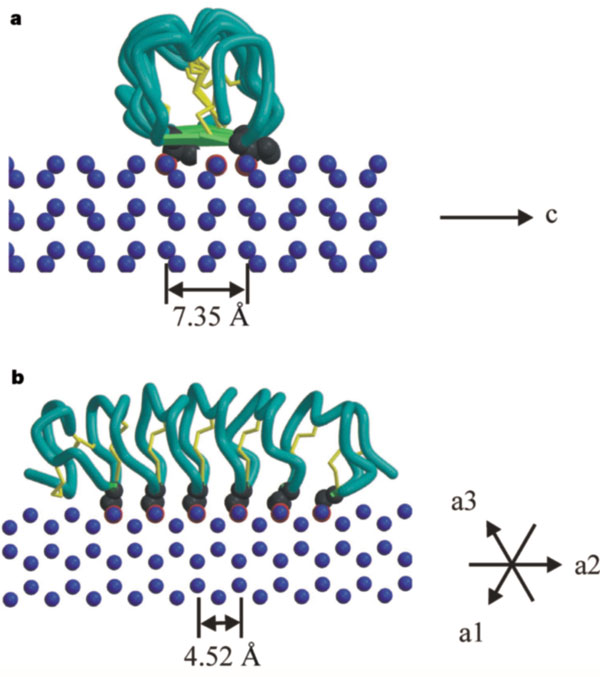
| - | ''Tm''AFP is an hyperactive antifreeze protein and its origin is the Tenebrio Mollitor beetle (Mealworm). These beetles are found in dark and cold areas, the temperature range in which it can survive can reach very low degrees- for example- the polarclimate. Due to ''Tm''AFP, Tenebrio Mollitor beetle has resistance against freezing - provides protection against physical and osmotic stresses. ''Tm''AFP is shaped like a slightly flattened cylinder 32Å in length, and 6.5X 14Å in the pseudo-rectangular cross section- the smallest Beta Hellix AFP known to date. | + | ''Tm''AFP is an hyperactive antifreeze protein and its origin is the Tenebrio Mollitor beetle (Mealworm). These beetles are found in dark and cold areas, the temperature range in which it can survive can reach very low degrees- for example- the polarclimate. Due to ''Tm''AFP, Tenebrio Mollitor beetle has resistance against freezing - provides protection against physical and osmotic stresses. ''Tm''AFP is shaped like a slightly flattened cylinder 32Å in length, and 6.5X 14Å in the pseudo-rectangular cross section- one of the smallest Beta Hellix AFP known to date. |
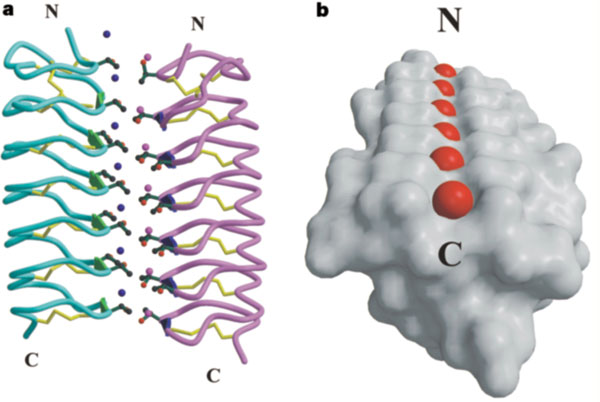
This is a <scene name='61/612804/Rainbow/1'>Rainbow</scene> view of the protein presenting the N terminal in blue and the C terminal in red. | This is a <scene name='61/612804/Rainbow/1'>Rainbow</scene> view of the protein presenting the N terminal in blue and the C terminal in red. | ||
| Line 12: | Line 12: | ||
The protein consists of 84 amino acids and the molecular weight is 8.4 kDA. | The protein consists of 84 amino acids and the molecular weight is 8.4 kDA. | ||
| - | ''Tm''AFP is protein rich of threonine and cystein in form of regular parallel beta-helix. It composed of 7 tandem repeats which consist of 12 amino acids-(TCTxSxxCxxAx). TCT (theonine, cystein, threonine) or ACT motifs are aligned to form a flat <scene name='61/612804/Beta_sheet/1'>Beta sheet</scene> along one side of the molecule the Beta sheets right handed which are the binding site of the protein. The rest of the tandem repeat forms the loop which enables very organized structure of the protein. Cysteine all over the tandem repeats, are pared to provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. Six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other <scene name='61/612804/2disulphide/1'>two disulphide bonds</scene> in the N-terminal region do not fit this pattern. The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop <scene name='61/612804/Hbond/2'>hydrogen bond</scene> connections between backbone CO and NH groups. | + | ''Tm''AFP is protein rich of threonine and cystein in form of regular parallel beta-helix. It composed of 7 tandem repeats which consist of 12 amino acids-(TCTxSxxCxxAx). TCT (theonine, cystein, threonine) or ACT motifs are aligned to form a flat <scene name='61/612804/Beta_sheet/1'>Beta sheet</scene> along one side of the molecule the Beta sheets right handed which are the binding site of the protein. The rest of the tandem repeat forms the loop which enables very organized structure of the protein. Cysteine all over the tandem repeats, are pared to provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. Six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other <scene name='61/612804/2disulphide/1'>two disulphide bonds</scene> in the N-terminal region do not fit this pattern and involved in a capping structure. The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop <scene name='61/612804/Hbond/2'>hydrogen bond</scene> connections between backbone CO and NH groups. |
| - | ''Tm''AFP is the smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core. The few Hydrophobic residues <scene name='61/612804/Hydrophobic/2'>val25, val34, phe58, tyr70</scene> have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge.<ref>DOI 10.1038/35018604</ref> | + | ''Tm''AFP is the one of smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core. The few Hydrophobic residues <scene name='61/612804/Hydrophobic/2'>val25, val34, phe58, tyr70</scene> have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge.<ref>DOI 10.1038/35018604</ref> |
Revision as of 12:13, 18 January 2015
| |||||||||||
References
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ Mitochondria as we don't know them. Tielens, Aloysius G.M et al. Trends in Biochemical Sciences , Volume 27 , Issue 11 , 564 - 572 doi:10.1016/S0968-0004(02)02193-X
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604