Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
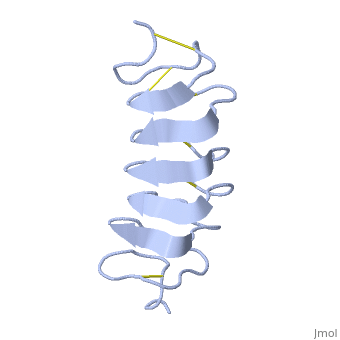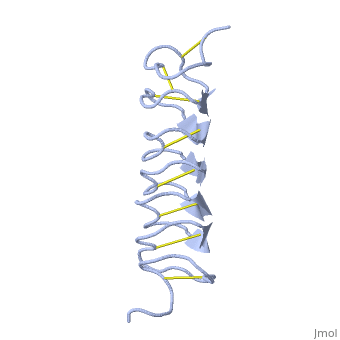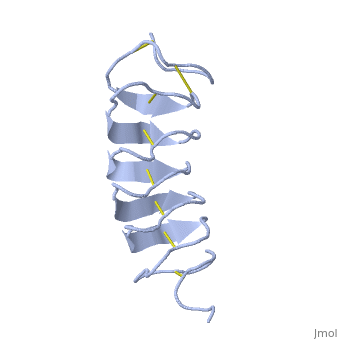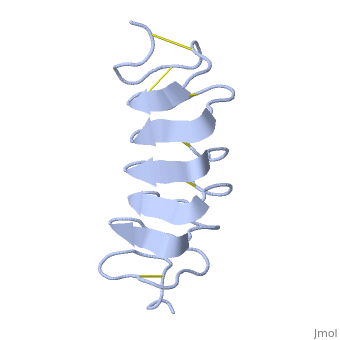
'''Lattice matching model for TmAFP binding to ice:''' | '''Lattice matching model for TmAFP binding to ice:''' | ||
| - | + | [[Image:406322ad.2.jpg|300px]] | |
| - | [[Image:406322ad.2.jpg]] | + | |
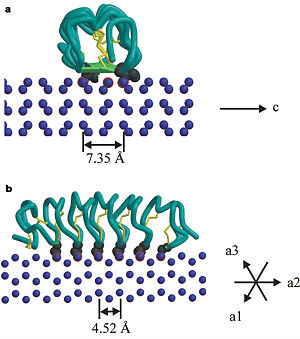
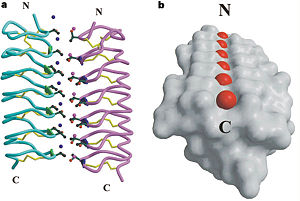
In solution the protein is monomeric but it crystallized as a <scene name='61/612804/Dimer/1'>dimer</scene>. The dimerization occurs along the surface of the beta sheets. The 2 units of the dimer do not directly interact with each other, the contact between them is mediated by highly ordered ranks of water which form hydrogen bonding with Threonine residue. Each water molecule forms two hydrogen bonds to the closer monomer and one to the distant monomer. The distance between two adjacent waters is 4.64±0.20Å the same distance as between Threonine residues 4.64±0.23Å. A good two dimensional match to the ice lattice including all 3 ranks of oxygen atoms (2 from Thr and 1 from water), implying that the ordered water molecules cold act as part of an ice surface and directly participate in the AFP-ice interaction. The regular array formed by this 3 ranks of oxygen atoms can be seen as a small piece of one layer thick ice to be incorporated into a large ice lattice. There is no need to readjust Threonine side chains, because they don’t present steric interference. <ref>DOI 10.1038/35018604</ref> | In solution the protein is monomeric but it crystallized as a <scene name='61/612804/Dimer/1'>dimer</scene>. The dimerization occurs along the surface of the beta sheets. The 2 units of the dimer do not directly interact with each other, the contact between them is mediated by highly ordered ranks of water which form hydrogen bonding with Threonine residue. Each water molecule forms two hydrogen bonds to the closer monomer and one to the distant monomer. The distance between two adjacent waters is 4.64±0.20Å the same distance as between Threonine residues 4.64±0.23Å. A good two dimensional match to the ice lattice including all 3 ranks of oxygen atoms (2 from Thr and 1 from water), implying that the ordered water molecules cold act as part of an ice surface and directly participate in the AFP-ice interaction. The regular array formed by this 3 ranks of oxygen atoms can be seen as a small piece of one layer thick ice to be incorporated into a large ice lattice. There is no need to readjust Threonine side chains, because they don’t present steric interference. <ref>DOI 10.1038/35018604</ref> | ||
'''Dimer of TmAFP and organization of external water:''' | '''Dimer of TmAFP and organization of external water:''' | ||
| - | [[Image:406322ac.2.jpg]] | + | [[Image:406322ac.2.jpg|300px]] |
Revision as of 06:24, 19 January 2015
| |||||||||||
References
- ↑ Liu K, Jia Z, Chen G, Tung C, Liu R. Systematic size study of an insect antifreeze protein and its interaction with ice. Biophys J. 2005 Feb;88(2):953-8. PMID:15713600 doi:http://dx.doi.org/10.1529/biophysj.104.051169
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ doi: https://dx.doi.org/10.1016/S0968-0004(01)02028-X
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604