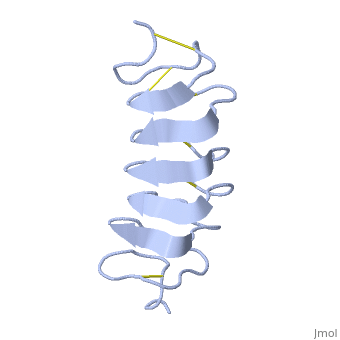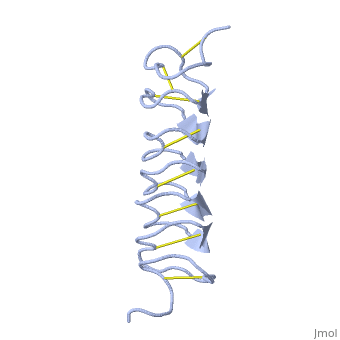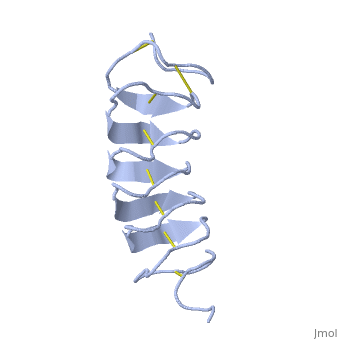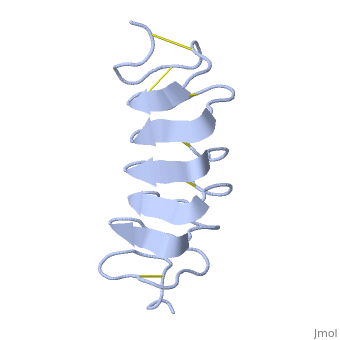
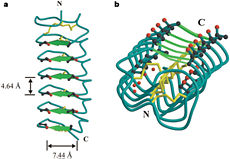
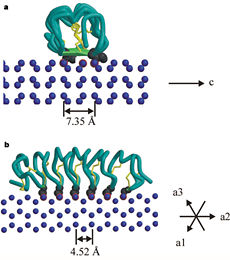
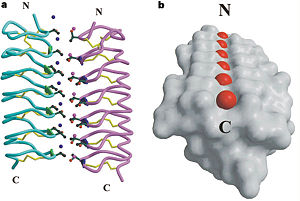
Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 43: | Line 43: | ||
== Function == | == Function == | ||
| - | The function of the ''Tm''AFP (And other Antifreeze protein) is Thermal Hysteresis(TH) .TH activity is occurs due to an adsorption inhibition mechanism that’s states that AFPs binds to ice surface and allow ice crystal growths only in surface regions between the bound AFP. Growing curvature causes an increase in surface energy, making the transformation of water into ice lass energetically favorable. Because of that the AFPs lower freezing temperature below the melting point. The difference between the melting and freezing point of the ice called thermal hysteresis activity.Due to the special structure of the protein, the TH activity can reach 6 | + | The function of the ''Tm''AFP (And other Antifreeze protein) is Thermal Hysteresis(TH) .TH activity is occurs due to an adsorption inhibition mechanism that’s states that AFPs binds to ice surface and allow ice crystal growths only in surface regions between the bound AFP. Growing curvature causes an increase in surface energy, making the transformation of water into ice lass energetically favorable. Because of that the AFPs lower freezing temperature below the melting point. The difference between the melting and freezing point of the ice called thermal hysteresis activity. |
| + | |||
| + | Due to the special structure of the protein, two dimensional surface that can bind to the two plans of ice, prism plane and basal plan, the TH activity can reach 6 Celsius degree (Hyperactive protein).In contrast moderate AFP (usually fish origin) has just one dimensional surface of threonine residue and thus can bind only to one plane of ice (the prism plan). E.g. <scene name='61/612804/Afp1/1'>Type I AFP</scene> from the fish winter flounder. Because of that The TH activity is much lower, maximum TH activity of moderate AFP is 1.5 Celsius degree | ||
<scene name='61/612804/Afp1/1'>TextToBeDisplayed</scene> | <scene name='61/612804/Afp1/1'>TextToBeDisplayed</scene> | ||
Revision as of 09:26, 19 January 2015
| |||||||||||
References
- ↑ Liu K, Jia Z, Chen G, Tung C, Liu R. Systematic size study of an insect antifreeze protein and its interaction with ice. Biophys J. 2005 Feb;88(2):953-8. PMID:15713600 doi:http://dx.doi.org/10.1529/biophysj.104.051169
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ doi: https://dx.doi.org/10.1016/S0968-0004(01)02028-X
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604