We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Tachyplesin
From Proteopedia
(Difference between revisions)
(Undo revision 2348791 by Michal Harel (Talk)) |
|||
| Line 6: | Line 6: | ||
== Structural highlights == | == Structural highlights == | ||
| - | + | Tachyplesin I is a 17-residue peptide containing six cationic residues with molecular weight 2,269 and isoelectric point (pI) of 9.93.<ref name=Chen>Chen, Yixin, et al. "RGD-Tachyplesin inhibits tumor growth." Cancer research 61.6 (2001): 2434-2438.</ref> | |
The amino acid sequence of the TP-I is NH₂-Lys-Trp-Cys-Phe-Arg-Val-Cys-Tyr-Arg-Gly-Ile-Cys-Tyr-Arg-Arg-Cys-Arg-CONH₂. | The amino acid sequence of the TP-I is NH₂-Lys-Trp-Cys-Phe-Arg-Val-Cys-Tyr-Arg-Gly-Ile-Cys-Tyr-Arg-Arg-Cys-Arg-CONH₂. | ||
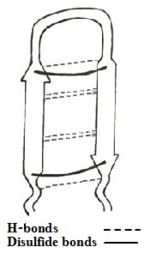
| - | [[Image:scheme1.jpg|150px|left|thumb|<b>Figure 1: | + | [[Image:scheme1.jpg|150px|left|thumb|<b>Figure 1: Simplified model of Tachyplesin I.</b>]] |
| Line 40: | Line 40: | ||
== A Cys Deleted Linear Analog == | == A Cys Deleted Linear Analog == | ||
| - | <scene name='67/671725/Cdt/1'> | + | <scene name='67/671725/Cdt/1'>Cysteine Deleted Tachyplesin</scene> (CDT) is a linear mutant lacking the cysteines and therefore lacking the disulfide bonds (NH₂-Lys-Trp-Phe-Arg-Val-Tyr-Arg-Gly-Ile-Tyr-Arg-Arg-Arg-CONH₂). It contains a broad spectrum of bactericidal activity with a reduced hemolytic property that stems from selective interactions with the negatively charged lipids including LPS. |
CDT has been demonstrated to markedly inhibit the growth of Gram negative and Gram positive bacterial strains akin to TP-I. But, minimum inhibitory concentration (MIC) values for CDT were found to be lower against [http://en.wikipedia.org/wiki/Escherichia_coli <i>Escherichia coli</i>] and [http://en.wikipedia.org/wiki/Listeria_monocytogenes <i>Listeria monocytogenes</i>] in comparison to the wild type TP-I peptide. | CDT has been demonstrated to markedly inhibit the growth of Gram negative and Gram positive bacterial strains akin to TP-I. But, minimum inhibitory concentration (MIC) values for CDT were found to be lower against [http://en.wikipedia.org/wiki/Escherichia_coli <i>Escherichia coli</i>] and [http://en.wikipedia.org/wiki/Listeria_monocytogenes <i>Listeria monocytogenes</i>] in comparison to the wild type TP-I peptide. | ||
| Line 47: | Line 47: | ||
CDT, like TP-I, has a β-turn in the <scene name='67/671725/Cdtturn/2'>same preserved residues</scene> in its LPS-bound structure. | CDT, like TP-I, has a β-turn in the <scene name='67/671725/Cdtturn/2'>same preserved residues</scene> in its LPS-bound structure. | ||
| - | The β-hairpin topology of CDT is sustained by the <scene name='67/671725/Cdthaipin/2'>unique packing interactions</scene> between the aromatic ring of Trp2 and the | + | The β-hairpin topology of CDT is sustained by the <scene name='67/671725/Cdthaipin/2'>unique packing interactions</scene> between the aromatic ring of Trp2 and the side-chain of nonpolar amino acid of Val5 and the cationic side-chain of residue Arg11. |
| - | There is a close proximity between residues <scene name='67/671725/Cdtnoe/1'>Trp2 and Ile9</scene>, supported by the [http://en.wikipedia.org/wiki/Nuclear_Overhauser_effect nuclear overhauser effects (NOEs)] involving [http://en.wikipedia.org/wiki/Indole indole] ring protons of Trp2 with | + | There is a close proximity between residues <scene name='67/671725/Cdtnoe/1'>Trp2 and Ile9</scene>, supported by the [http://en.wikipedia.org/wiki/Nuclear_Overhauser_effect nuclear overhauser effects (NOEs)] involving [http://en.wikipedia.org/wiki/Indole indole] ring protons of Trp2 with side-chain proton of Ile9. These packing interactions have rendered an approximate anti-parallel orientation of the hairpin structure of CDT in presence of LPS. |
The β-hairpin like structure of CDT displays an extended <font color='darkblue'>positively charged</font> surface patch of <scene name='67/671725/Cdtr4r7r12r13/1'>residues Arg 4, 7, 12 and 13</scene>. These positively charged basic residues interacts with the anionic phosphate groups of LPS with the help of salt bridges and/or hydrogen bonds. | The β-hairpin like structure of CDT displays an extended <font color='darkblue'>positively charged</font> surface patch of <scene name='67/671725/Cdtr4r7r12r13/1'>residues Arg 4, 7, 12 and 13</scene>. These positively charged basic residues interacts with the anionic phosphate groups of LPS with the help of salt bridges and/or hydrogen bonds. | ||
| Line 54: | Line 54: | ||
== Mode of action == | == Mode of action == | ||
| - | TP-I has affinity to LPS and also has ability to permeabilize the cell membrane of pathogens. TP-I primary and critical target is the cell membrane.<ref name=Hong>Hong, Jun, et al. "Mechanism of | + | TP-I has affinity to LPS and also has ability to permeabilize the cell membrane of pathogens. TP-I primary and critical target is the cell membrane.<ref name=Hong>Hong, Jun, et al. "Mechanism of Tachyplesin I injury to bacterial membranes and intracellular enzymes, determined by laser confocal scanning microscopy and flow cytometry." Microbiological research (2014).</ref>Docking model suggests strong affinity between TP-I and LPS; gained by interaction between cationic residues of TP-I with phosphate group and saccharides of LPS. Furthermore, interaction between hydrophobic residues of TP-I with acyl chains of LPS strengthens the TP-I/LPS interaction. The binding of TP-I/LPS neutralizes LPS, which is widely considered as endotoxin, and disrupts membrane function. |
In addition to LPS binding, footpriting analysis has revealed the binding of TP-I to DNA by interacting specifically in minor groove of DNA duplex. The interaction between TP-I and DNA is contributed by secondary structure of the peptide which contains an antiparallel beta-sheet constrained by two disulfide bridges and connected by β-turn <ref name=Yonezawa>PMID:1372516</ref>. | In addition to LPS binding, footpriting analysis has revealed the binding of TP-I to DNA by interacting specifically in minor groove of DNA duplex. The interaction between TP-I and DNA is contributed by secondary structure of the peptide which contains an antiparallel beta-sheet constrained by two disulfide bridges and connected by β-turn <ref name=Yonezawa>PMID:1372516</ref>. | ||
| Line 69: | Line 69: | ||
The potential mechanism of <i>E. coli</i> membrane disruption by TP-I is the induction of macromolecule leakage into the cytoplasm and the release of potassium ions, leading to an increase in inner permeability, the formation of a toroidal pore, the neutralization of LPS, and the disruption of the permeability barrier of the outer membrane. TP-I killed <i>E. coli</i> mainly through cell membrane damage and intracellular esterase inactivation dependent on concentration. | The potential mechanism of <i>E. coli</i> membrane disruption by TP-I is the induction of macromolecule leakage into the cytoplasm and the release of potassium ions, leading to an increase in inner permeability, the formation of a toroidal pore, the neutralization of LPS, and the disruption of the permeability barrier of the outer membrane. TP-I killed <i>E. coli</i> mainly through cell membrane damage and intracellular esterase inactivation dependent on concentration. | ||
| - | In food production, requirements must be met for producing high quality food with minimal microbial contamination, and the determination of microbial viability based on different physiological and metabolic parameters is critical for acceptable sterilization. Therefore, the presence of injured, metabolically active bacteria is a very important aspect to consider in food production and for clinical applications. | + | In food production, requirements must be met for producing high quality food with minimal microbial contamination, and the determination of microbial viability based on different physiological and metabolic parameters is critical for acceptable sterilization. Therefore, the presence of injured, metabolically active bacteria is a very important aspect to consider in food production and for clinical applications. Sub lethally injured cells might be repaired under suitable conditions. If TP-I is applied as a clinical treatment at a lower concentration than the [http://en.wikipedia.org/wiki/Minimum_inhibitory_concentration MIC] over a long period of time, drug resistance could develop.<ref name=Hong>Hong, Jun, et al. "Mechanism of tachyplesin I injury to bacterial membrane and intracellular enzymes, determined by laser confocal scanning microscopy and flow cytometry." Microbiological research (2014).</ref> |
== Possible Function as anti-tumor peptide == | == Possible Function as anti-tumor peptide == | ||
| - | The cationic nature of Tachyplesin allows it to interact with anionic phospholipids present in the bacterial membrane and thereby disrupting membrane function. Besides this, the structural nature of Tachyplesin also highlights its antitumor properties. Since it can interact with the membrance of prokaryotic cell, it is likely that TP-I can also interact with the mitochondrial membrane of eukaryotic cells. Mitochondria are widely believed to have evolved from prokaryotic cells, that have established a symbiotic relationship with the primitive eukaryotic cell which signifies the structural similarity of | + | The cationic nature of Tachyplesin allows it to interact with anionic phospholipids present in the bacterial membrane and thereby disrupting membrane function. Besides this, the structural nature of Tachyplesin also highlights its antitumor properties. Since it can interact with the membrance of prokaryotic cell, it is likely that TP-I can also interact with the mitochondrial membrane of eukaryotic cells. Mitochondria are widely believed to have evolved from prokaryotic cells, that have established a symbiotic relationship with the primitive eukaryotic cell which signifies the structural similarity of mitochondrial and prokaryotic membranes. |
| - | It was found that the synthetic | + | It was found that the synthetic Tachyplesin conjugated to the integrin homing domain (RGD-Tachyplesin) can inhibit the [http://en.wikipedia.org/wiki/Cell_growth proliferation] of TSU tumor cells [http://en.wikipedia.org/wiki/Prostate_cancer prostate cancer] and B16 [http://en.wikipedia.org/wiki/Melanoma melanoma] cells as well as [http://en.wikipedia.org/wiki/Endothelium endothelial cells] in a dose-dependent manner <i>in vitro</i> and reduce tumor growth <i>in vivo</i> by inducing [http://en.wikipedia.org/wiki/Apoptosis apoptosis].<ref name=Chen>PMID:11289111</ref>. Besides this RGD-Tachyplesin can activate caspases and induce Fas ligand, which are the markers for programmed cell death (PCD). Collectively, suppression of tumor associated cell and induction of programmed cell death will eventually act as therapy for cancer and tumor cells. |
</StructureSection> | </StructureSection> | ||
Revision as of 12:02, 19 January 2015
Introduction
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Laederach A, Andreotti AH, Fulton DB. Solution and micelle-bound structures of tachyplesin I and its active aromatic linear derivatives. Biochemistry. 2002 Oct 15;41(41):12359-68. PMID:12369825
- ↑ 2.0 2.1 Chen, Yixin, et al. "RGD-Tachyplesin inhibits tumor growth." Cancer research 61.6 (2001): 2434-2438.
- ↑ Nakamura, Takanori, et al. "Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure." Journal of Biological Chemistry 263.32 (1988): 16709-16713
- ↑ Kushibiki T, Kamiya M, Aizawa T, Kumaki Y, Kikukawa T, Mizuguchi M, Demura M, Kawabata SI, Kawano K. Interaction between tachyplesin I, an antimicrobial peptide derived from horseshoe crab, and lipopolysaccharide. Biochim Biophys Acta. 2014 Jan 2;1844(3):527-534. doi:, 10.1016/j.bbapap.2013.12.017. PMID:24389234 doi:http://dx.doi.org/10.1016/j.bbapap.2013.12.017
- ↑ 5.0 5.1 Saravanan R, Mohanram H, Joshi M, Domadia PN, Torres J, Ruedl C, Bhattacharjya S. Structure, activity and interactions of the cysteine deleted analog of tachyplesin-1 with lipopolysaccharide micelle: Mechanistic insights into outer-membrane permeabilization and endotoxin neutralization. Biochim Biophys Acta. 2012 Mar 23;1818(7):1613-1624. PMID:22464970 doi:10.1016/j.bbamem.2012.03.015
- ↑ 6.0 6.1 6.2 Hong, Jun, et al. "Mechanism of Tachyplesin I injury to bacterial membranes and intracellular enzymes, determined by laser confocal scanning microscopy and flow cytometry." Microbiological research (2014).
- ↑ Yonezawa A, Kuwahara J, Fujii N, Sugiura Y. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry. 1992 Mar 24;31(11):2998-3004. PMID:1372516
- ↑ Lipsky A, Cohen A, Ion A, Yedidia I. Genetic transformation of Ornithogalum via particle bombardment and generation of Pectobacterium carotovorum-resistant plants. Plant Sci. 2014 Nov;228:150-8. doi: 10.1016/j.plantsci.2014.02.002. Epub 2014 Feb, 12. PMID:25438795 doi:http://dx.doi.org/10.1016/j.plantsci.2014.02.002
Quiz
Proteopedia Page Contributors and Editors (what is this?)
Shulamit Idzikowski, Janak Raj Joshi, Michal Harel, Alexander Berchansky, Joel L. Sussman, Angel Herraez, Jaime Prilusky