The Rv0899 ArfA protein belongs to the OmpA (outer membrane protein A) family of outer membrane proteins and has been proposed to act as an outer membrane porin and contributes to the bacterium's adaptation to the acidic environment.The protein Rv0899 ArfA is restricted to pathogenic Mycobacterium associated with tuberculosis and, thus, is an attractive candidate for the development of anti-tuberculosis chemotherapeutic agents.
The deletion of this gene impairs the uptake of some water-soluble substances, such as serine, glucose, and glycerol.
Structure Section
The 326-residue Rv0899 ArfA contains three domains: an N-terminal domain (M domain) (residues 1-72) which includes a sequence of 20 hydrophobic amino acids required for membrane translocation.
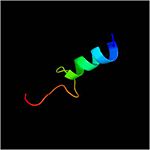
Residues form a mixed alpha/beta-globular structure, encompassing two independently folded modules corresponding to the B and C domains connected by a flexible linker.
The central B domain ( ) with three parallel/antiparallel alpha-helices packed against six parallel/antiparallel beta-strands that form a flat beta-sheet.
The B domain has homology with conserved putative (bacterial OsmY and nodulation), superfamily domains and conserved_Gly95_and_Gly164
[1].
is hydrophobic, while the exterior is polar and predominantly acidic.
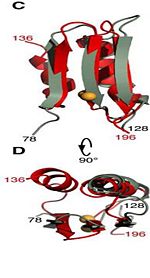
The C domain has homology to the OmpA-C-like superfamily of periplasmic peptidoglycan-binding sequences, found in several types of bacterial membrane proteins.The C domain of wild-type ArfA into four β-strands and four α-helices, arranged in the topological order αβαβαβαβ. Three parallel (β1, β2, β3) and one antiparallel (β4) β-strands form a four-stranded β-sheet (β1–β4-β2–β3) that packs against three α-helices (α1, α2, α3), while a fourth helix (α4) extends from the N-terminus of β4. A disulfide bondbetween C208 and C250 connects the N-terminus of α1 to the C-terminus of α2 and stabilizes the structure.
Function
Using NMR chemical shift perturbation and isothermal calorimetric titration assays, Rv0899 was able to interact with , which may indicate a role for Rv0899 in the process of Zn(2+) acquisition.
[1]
Rv0899 contribute to the bacterium's adaptation to the acidic environment of the phagosome[2] during infection [ARFA_MYCTU]. The protein encoded by an ammonia release facilator operon that is necessary for rapid ammonia secretion, pH neutralization and adaptation to acidic environments in vitro.
It exhibits pH-dependent conformational dynamics and a more ordered structure at , which could be related to its acid stress response.
Its functions in acid stress protection and [peptidoglycan][3] binding site suggest a link between the acid stress response and the physicochemical properties of the mycobacterial cell wall. These residues are strictly conserved in the OmpA -like family [2].
Two M. tuberculosis H37Rv genes (Rv0900 and Rv0901) adjacent to Rv0899 also encode putative membrane proteins, and are found exclusively in association with Rv0899 in the same pathogenic mycobacteria, suggesting that the three may constitute an operon dedicated to a common function. The operon is necessary for rapid ammonia secretion and adaptation of M. tuberculosis to acidic environments in vitro but not in mice.
Asparagine is the primary ammonia source for Mycobacterium tuberculosis H37Rv at acidic pH [3]. The amino acid pair ,located at the end of α1 and preceding L3, undergoes in-vitro deamidation, a pH-dependent reaction whereby Asn is converted to Asp and ammonia is released. Asparagine residues preceding glycine, and situated in conformationally flexible regions of proteins, are frequently deamidated, with potentially significant consequences for protein regulation and function. In the case of Rv0899, deamidation and the concomitant release of ammonia could have important consequences for the acid adaptation function of the protein. [4]
Peptidoglycan binding site
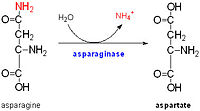
Image:Rv0899 memb arch.jpg
References
- ↑ Li J, Shi C, Gao Y, Wu K, Shi P, Lai C, Chen L, Wu F, Tian C. Structural Studies of Mycobacterium tuberculosis Rv0899 Reveal a Monomeric Membrane-Anchoring Protein with Two Separate Domains. J Mol Biol. 2011 Nov 15. PMID:22108166 doi:10.1016/j.jmb.2011.11.016
- ↑ Yao Y, Barghava N, Kim J, Niederweis M, Marassi FM. Molecular Structure and Peptidoglycan Recognition of Mycobacterium tuberculosis ArfA (Rv0899). J Mol Biol. 2012 Feb 17;416(2):208-20. Epub 2011 Dec 21. PMID:22206986 doi:10.1016/j.jmb.2011.12.030
- ↑ Song H, Huff J, Janik K, Walter K, Keller C, Ehlers S, Bossmann SH, Niederweis M. Expression of the ompATb operon accelerates ammonia secretion and adaptation of Mycobacterium tuberculosis to acidic environments. Mol Microbiol. 2011 May;80(4):900-18. doi: 10.1111/j.1365-2958.2011.07619.x. Epub, 2011 Mar 16. PMID:21410778 doi:http://dx.doi.org/10.1111/j.1365-2958.2011.07619.x
- ↑ Teriete P, Yao Y, Kolodzik A, Yu J, Song H, Niederweis M, Marassi FM. Mycobacterium tuberculosis Rv0899 Adopts a Mixed alpha/beta-Structure and Does Not Form a Transmembrane beta-Barrel. Biochemistry. 2010 Mar 10. PMID:20199110 doi:10.1021/bi100158s





