Mycobacterium tuberculosis ArfA Rv0899
From Proteopedia
(Difference between revisions)
| Line 36: | Line 36: | ||
<ref>PMID: 22108166 </ref> | <ref>PMID: 22108166 </ref> | ||
| - | 2. Bacterium's adaptation to the acidic environment of the phagosome[http://en.wikipedia.org/wiki/Phagosome] during infection. | + | 2. Bacterium's adaptation to the acidic environment of the phagosome[http://en.wikipedia.org/wiki/Phagosome] during infection. |
| + | Rv0899 protein encoded by an ammonia release facilator operon that is necessary for rapid ammonia secretion, pH neutralization and adaptation to acidic environments in vitro. Two M. tuberculosis H37Rv genes (Rv0900 [[http://www.uniprot.org/uniprot/P9WJG7 ARFB_MYCTU]] and Rv0901 [[http://www.uniprot.org/uniprot/P9WJG5 ARFC_MYCTU]] ) adjacent to Rv0899 also encode putative membrane proteins, and are found exclusively in association with Rv0899 in the same pathogenic mycobacteria, suggesting that the three may constitute an operon dedicated to a common function. | ||
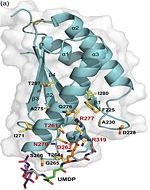
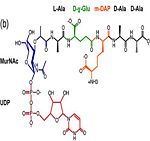
Asparagine is the primary ammonia source for Mycobacterium tuberculosis H37Rv at acidic pH <ref>PMID: 21410778 </ref>. The amino acid pair <scene name='61/612805/Asn111_and_gly112/1'> Asn111-Gly112 </scene>, located at the end of α1 and preceding L3, undergoes in-vitro deamidation, a pH-dependent reaction whereby Asn is converted to Asp and ammonia is released. Asparagine residues preceding glycine, and situated in conformationally flexible regions of proteins, are frequently deamidated, with potentially significant consequences for protein regulation and function. In the case of Rv0899, deamidation and the concomitant release of ammonia could have important consequences for the acid adaptation function of the protein. <ref>PMID: 20199110</ref> | Asparagine is the primary ammonia source for Mycobacterium tuberculosis H37Rv at acidic pH <ref>PMID: 21410778 </ref>. The amino acid pair <scene name='61/612805/Asn111_and_gly112/1'> Asn111-Gly112 </scene>, located at the end of α1 and preceding L3, undergoes in-vitro deamidation, a pH-dependent reaction whereby Asn is converted to Asp and ammonia is released. Asparagine residues preceding glycine, and situated in conformationally flexible regions of proteins, are frequently deamidated, with potentially significant consequences for protein regulation and function. In the case of Rv0899, deamidation and the concomitant release of ammonia could have important consequences for the acid adaptation function of the protein. <ref>PMID: 20199110</ref> | ||
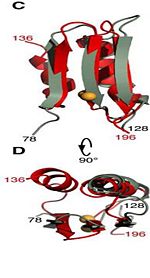
| - | It exhibits pH-dependent conformational dynamics <scene name='61/612805/D236_before_mutation/1'>in neutral pH</scene> and a more ordered structure at <scene name='61/612805/D236a_after_mut/3'>acidic pH</scene>, which could be related to its acid stress response. | + | It exhibits pH-dependent conformational dynamics 232, 225, 240, 244, 281, 285 <scene name='61/612805/D236_before_mutation/1'>in neutral pH (D236) </scene> and a more ordered structure at <scene name='61/612805/D236a_after_mut/3'>acidic pH (D236A) </scene>, which could be related to its acid stress response. |
[[Image:Asparaginase-reaction.jpg|250px]] | [[Image:Asparaginase-reaction.jpg|250px]] | ||
Revision as of 17:34, 21 January 2015
| |||||||||||