Mycobacterium tuberculosis ArfA Rv0899
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
==Introduction== | ==Introduction== | ||
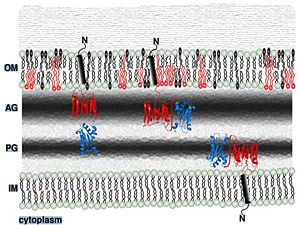
| - | The Rv0899 ArfA protein belongs to the OmpA (outer membrane protein A) family of outer membrane proteins and has been proposed to act as an outer membrane [[porin]] with ion channel activity and contributes to the bacterium's adaptation to the acidic environment <ref>PMID: 21117233 </ref>. | + | The Rv0899 ArfA protein belongs to the OmpA (outer membrane protein A) family of outer membrane proteins and has been proposed to act as an outer membrane [[porin]] with ion channel activity and contributes to the bacterium's adaptation to the acidic environment in phagosome [http://en.wikipedia.org/wiki/Phagosome]during infection <ref>PMID: 21117233 </ref>. |
The deletion of this gene impairs the uptake of some water-soluble substances, such as serine, glucose, and glycerol <ref>PMID: 12366842 </ref>. | The deletion of this gene impairs the uptake of some water-soluble substances, such as serine, glucose, and glycerol <ref>PMID: 12366842 </ref>. | ||
| Line 19: | Line 19: | ||
Residues <scene name='61/612805/N-c_rainbow/1'>73-326</scene> form a mixed alpha/beta-globular structure, encompassing two independently folded modules corresponding to the B and C domains connected by a flexible linker. | Residues <scene name='61/612805/N-c_rainbow/1'>73-326</scene> form a mixed alpha/beta-globular structure, encompassing two independently folded modules corresponding to the B and C domains connected by a flexible linker. | ||
| - | The central B domain ( <scene name='61/612805/B_domain/1'>residues 73-200</scene> ) <scene name='61/612805/Sheet_and_helix/1'> folds </scene> with three parallel/antiparallel alpha-helices packed against six parallel/antiparallel beta-strands that form a flat beta-sheet. | + | The central B domain ( <scene name='61/612805/B_domain/1'>residues 73-200</scene> ) <scene name='61/612805/Sheet_and_helix/1'> folds </scene> with three parallel/antiparallel alpha-helices packed against six parallel/antiparallel beta-strands that form a flat beta-sheet. The B domain has homology with conserved putative <scene name='61/612805/Conserved_g95_and_g164_in_bon/2'>lipid-binding BON</scene>(bacterial OsmY and nodulation), superfamily domains and conserved_Gly95_and_Gly164 [http://www.ebi.ac.uk/interpro/entry/IPR014004]. |
| - | The B domain has homology with conserved putative <scene name='61/612805/Conserved_g95_and_g164_in_bon/2'>lipid-binding BON</scene> | + | <scene name='61/612805/Surface/1'>The core </scene> is hydrophobic, while the exterior is polar and predominantly acidic. The two subdomains are symmetric about α2, and their backbone atoms can be aligned with a RMSD of 1.8Å, by performing a 180° rotation of either one around an axis normal to the 6-stranded β-sheet. |
| - | [http://www.ebi.ac.uk/interpro/entry/IPR014004]. | + | |
| - | <scene name='61/612805/Surface/1'>The core </scene> is hydrophobic, while the exterior is polar and predominantly acidic. | + | |
| Line 29: | Line 27: | ||
The C domain <scene name='61/612805/C_domain/1'>(residues 201-326)</scene> has homology to the OmpA-C-like superfamily of periplasmic peptidoglycan-binding sequences, found in several types of bacterial membrane proteins.The C domain of wild-type ArfA <scene name='61/612805/C_domain/3'></scene> <scene name='61/612805/C_domain_1/1'> folds </scene> into four β-strands and four α-helices.Three parallel (β1, β2, β3) and one antiparallel (β4) β-strands form a four-stranded β-sheet (β1–β4-β2–β3) that packs against three α-helices (α1, α2, α3), while a fourth helix (α4) extends from the N-terminus of β4. <scene name='61/612805/C_domain_stabilization/2'> A disulfide bond between C208 and C250 and hydrophobic bonds between side chains and hydrogen</scene> | The C domain <scene name='61/612805/C_domain/1'>(residues 201-326)</scene> has homology to the OmpA-C-like superfamily of periplasmic peptidoglycan-binding sequences, found in several types of bacterial membrane proteins.The C domain of wild-type ArfA <scene name='61/612805/C_domain/3'></scene> <scene name='61/612805/C_domain_1/1'> folds </scene> into four β-strands and four α-helices.Three parallel (β1, β2, β3) and one antiparallel (β4) β-strands form a four-stranded β-sheet (β1–β4-β2–β3) that packs against three α-helices (α1, α2, α3), while a fourth helix (α4) extends from the N-terminus of β4. <scene name='61/612805/C_domain_stabilization/2'> A disulfide bond between C208 and C250 and hydrophobic bonds between side chains and hydrogen</scene> | ||
| - | stabilizes the structure. | + | stabilizes the structure . |
==Putative functions== | ==Putative functions== | ||
| Line 36: | Line 34: | ||
<ref>PMID: 22108166 </ref> | <ref>PMID: 22108166 </ref> | ||
| - | 2. Bacterium's adaptation to the acidic environment of the phagosome | + | 2. Bacterium's adaptation to the acidic environment of the phagosome during infection. |
Rv0899 protein encoded by an ammonia release facilator operon that is necessary for rapid ammonia secretion, pH neutralization and adaptation to acidic environments in vitro. Two M. tuberculosis H37Rv genes (Rv0900 [[http://www.uniprot.org/uniprot/P9WJG7 ARFB_MYCTU]] and Rv0901 [[http://www.uniprot.org/uniprot/P9WJG5 ARFC_MYCTU]] ) adjacent to Rv0899 also encode putative membrane proteins, and are found exclusively in association with Rv0899 in the same pathogenic mycobacteria, suggesting that the three may constitute an operon dedicated to a common function. | Rv0899 protein encoded by an ammonia release facilator operon that is necessary for rapid ammonia secretion, pH neutralization and adaptation to acidic environments in vitro. Two M. tuberculosis H37Rv genes (Rv0900 [[http://www.uniprot.org/uniprot/P9WJG7 ARFB_MYCTU]] and Rv0901 [[http://www.uniprot.org/uniprot/P9WJG5 ARFC_MYCTU]] ) adjacent to Rv0899 also encode putative membrane proteins, and are found exclusively in association with Rv0899 in the same pathogenic mycobacteria, suggesting that the three may constitute an operon dedicated to a common function. | ||
Asparagine is the primary ammonia source for Mycobacterium tuberculosis H37Rv at acidic pH <ref>PMID: 21410778 </ref>. The amino acid pair <scene name='61/612805/Asn111_and_gly112/1'> Asn111-Gly112 </scene>, located at the end of α1 and preceding L3, undergoes in-vitro deamidation, a pH-dependent reaction whereby Asn is converted to Asp and ammonia is released. Asparagine residues preceding glycine, and situated in conformationally flexible regions of proteins, are frequently deamidated, with potentially significant consequences for protein regulation and function. In the case of Rv0899, deamidation and the concomitant release of ammonia could have important consequences for the acid adaptation function of the protein. <ref>PMID: 20199110</ref> | Asparagine is the primary ammonia source for Mycobacterium tuberculosis H37Rv at acidic pH <ref>PMID: 21410778 </ref>. The amino acid pair <scene name='61/612805/Asn111_and_gly112/1'> Asn111-Gly112 </scene>, located at the end of α1 and preceding L3, undergoes in-vitro deamidation, a pH-dependent reaction whereby Asn is converted to Asp and ammonia is released. Asparagine residues preceding glycine, and situated in conformationally flexible regions of proteins, are frequently deamidated, with potentially significant consequences for protein regulation and function. In the case of Rv0899, deamidation and the concomitant release of ammonia could have important consequences for the acid adaptation function of the protein. <ref>PMID: 20199110</ref> | ||
Revision as of 13:53, 22 January 2015
| |||||||||||