We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Tachyplesin
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
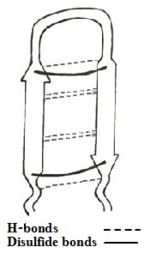
| - | The sequence adapts an antiparallel β-sheet (hairpin) conformation in solution, with a <scene name='67/671725/Beta_turn_tp-1/2'>β-turn</scene> for the centrally located residues <scene name='67/671725/Tyrargglyile/3'>Tyr-Arg-Gly-Ile</scene>, stabilized by two cross-strand <scene name='67/671725/Disulfide_bonds/4'> disulfide bonds </scene> between Cys³-Cys¹⁶ and Cys⁷-Cys¹²<ref name=Saravanan>PMID:22464970</ref><ref name=Nakamura>Nakamura, Takanori, et al. "Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (''Tachypleus tridentatus''). Isolation and chemical structure." Journal of Biological Chemistry 263.32 (1988): 16709-16713</ref>, and [http://en.wikipedia.org/wiki/Protein_primary_structure C-terminus amidation]. In addition there are H-bonds and aromatic rings stacking interactions which helps stabilize the hairpin loop structure of the peptide. Among all the existing interactions, cysteine bridge being considered as the principal contributor of the structure, three linear derivatives of TP-I (<scene name='67/671725/1ma4/3'>TPY4</scene>, TPF4 and TPA4) were created, in which the bridging cysteine residues are uniformly replaced with tyrosine, phenylalanine, and alanine, respectively<ref name=Laederach>PMID:12369825</ref><ref name=Kushibiki>PMID:24389234</ref>. The linear derivatives of TP-I are mentioned below: | + | The sequence adapts an antiparallel β-sheet (hairpin) conformation in solution, with a <scene name='67/671725/Beta_turn_tp-1/2'>β-turn</scene> for the centrally located residues <scene name='67/671725/Tyrargglyile/3'>Tyr-Arg-Gly-Ile</scene>, stabilized by two cross-strand <scene name='67/671725/Disulfide_bonds/4'> disulfide bonds </scene> between Cys³-Cys¹⁶ and Cys⁷-Cys¹²<ref name=Saravanan>PMID:22464970</ref><ref name=Nakamura>Nakamura, Takanori, et al. "Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (''Tachypleus tridentatus''). Isolation and chemical structure." Journal of Biological Chemistry 263.32 (1988): 16709-16713</ref>, and [http://en.wikipedia.org/wiki/Protein_primary_structure C-terminus amidation]. In addition there are H-bonds and aromatic rings stacking interactions which helps stabilize the hairpin loop structure of the peptide. |
| + | |||
| + | TP-I undergoes a conformational change in the <scene name='67/671725/Tp_i_in_the_presence_of_lps/1'>presence of LPS </scene>. The backbone of the polypeptide becomes <scene name='67/671725/Conformation_change/5'>more rigid and twisted in the presence of LPS, than in the presence of water</scene>, making it more stable. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Derivatives and Analouge == | ||
| + | Among all the existing interactions, cysteine bridge being considered as the principal contributor of the structure, three linear derivatives of TP-I (<scene name='67/671725/1ma4/3'>TPY4</scene>, TPF4 and TPA4) were created, in which the bridging cysteine residues are uniformly replaced with tyrosine, phenylalanine, and alanine, respectively<ref name=Laederach>PMID:12369825</ref><ref name=Kushibiki>PMID:24389234</ref>. The linear derivatives of TP-I are mentioned below: | ||
[[Image:derivatives.jpg]] | [[Image:derivatives.jpg]] | ||
| - | Of those 3 linear derivatives of TP-I, NMR structural investigation had shown | + | Of those 3 linear derivatives of TP-I, NMR structural investigation had shown TPA4 to be unstructured in solution. Also, TPA4 was inactive in terms of antimicrobial activity. In contrast, TPY4 and TPF4 adapts hairpin loop and also had antimicrobial properties, typical to TP-I. Therefore, the hairpin properties of the peptide seems to be important for recognition of lipopolysaccharides and its biological activities. |
| - | + | ||
| - | + | ||
| - | + | Besides replacement of cysteines, deletions was performed in TP-I which yielded <scene name='67/671725/Cdt/1'>Cysteine Deleted Tachyplesin</scene> (CDT). CTD is a linear mutant lacking the cysteines and therefore lacking the disulfide bonds (NH₂-Lys-Trp-Phe-Arg-Val-Tyr-Arg-Gly-Ile-Tyr-Arg-Arg-Arg-CONH₂). It contains a broad spectrum of bactericidal activity with a reduced hemolytic property that stems from selective interactions with the negatively charged lipids including LPS. | |
| - | <scene name='67/671725/Cdt/1'>Cysteine Deleted Tachyplesin</scene> (CDT) is a linear mutant lacking the cysteines and therefore lacking the disulfide bonds (NH₂-Lys-Trp-Phe-Arg-Val-Tyr-Arg-Gly-Ile-Tyr-Arg-Arg-Arg-CONH₂). It contains a broad spectrum of bactericidal activity with a reduced hemolytic property that stems from selective interactions with the negatively charged lipids including LPS. | + | |
CDT has been demonstrated to markedly inhibit the growth of Gram negative and Gram positive bacterial strains akin to TP-I. But, minimum inhibitory concentration (MIC) values for CDT were found to be lower against [http://en.wikipedia.org/wiki/Escherichia_coli <i>Escherichia coli</i>] and [http://en.wikipedia.org/wiki/Listeria_monocytogenes <i>Listeria monocytogenes</i>] in comparison to the wild type TP-I peptide. | CDT has been demonstrated to markedly inhibit the growth of Gram negative and Gram positive bacterial strains akin to TP-I. But, minimum inhibitory concentration (MIC) values for CDT were found to be lower against [http://en.wikipedia.org/wiki/Escherichia_coli <i>Escherichia coli</i>] and [http://en.wikipedia.org/wiki/Listeria_monocytogenes <i>Listeria monocytogenes</i>] in comparison to the wild type TP-I peptide. | ||
Revision as of 19:35, 22 January 2015
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Shulamit Idzikowski, Janak Raj Joshi, Michal Harel, Alexander Berchansky, Joel L. Sussman, Angel Herraez, Jaime Prilusky