This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Tachyplesin
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
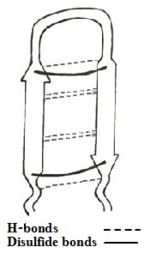
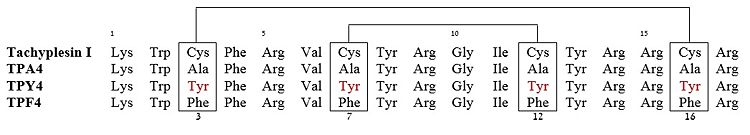
Of those 3 linear derivatives of TP-I, NMR structural investigation had shown TPA4 to be unstructured in solution. Also, TPA4 was inactive in terms of antimicrobial activity. In contrast, TPY4 and TPF4 adapts hairpin loop and also had antimicrobial properties, typical to TP-I. Therefore, the hairpin properties of the peptide seems to be important for recognition of lipopolysaccharides and its biological activities. | Of those 3 linear derivatives of TP-I, NMR structural investigation had shown TPA4 to be unstructured in solution. Also, TPA4 was inactive in terms of antimicrobial activity. In contrast, TPY4 and TPF4 adapts hairpin loop and also had antimicrobial properties, typical to TP-I. Therefore, the hairpin properties of the peptide seems to be important for recognition of lipopolysaccharides and its biological activities. | ||
| - | Besides replacement of cysteines, deletions was also performed in TP-I which yielded <scene name='67/671725/Cdt/1'>Cysteine Deleted Tachyplesin</scene> (CDT). Thus, CTD | + | Besides replacement of cysteines, deletions was also performed in TP-I which yielded <scene name='67/671725/Cdt/1'>Cysteine Deleted Tachyplesin</scene> (CDT). Thus, CTD with sequence NH₂-Lys-Trp-Phe-Arg-Val-Tyr-Arg-Gly-Ile-Tyr-Arg-Arg-Arg-CONH₂ did not have disulphide linkage, but was found to have broad spectrum of bactericidal activity. Specifically, CDT has been demonstrated to markedly inhibit the growth of [http://en.wikipedia.org/wiki/Escherichia_coli <i>Escherichia coli</i>] and [http://en.wikipedia.org/wiki/Listeria_monocytogenes <i>Listeria monocytogenes</i>] akin to TP-I, even with lower minimum inhibitory concentration (MIC) values. |
<b><u> CDT Structure </u></b> | <b><u> CDT Structure </u></b> | ||
Revision as of 10:15, 24 January 2015
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Laederach A, Andreotti AH, Fulton DB. Solution and micelle-bound structures of tachyplesin I and its active aromatic linear derivatives. Biochemistry. 2002 Oct 15;41(41):12359-68. PMID:12369825
- ↑ 2.0 2.1 Chen, Yixin, et al. "RGD-Tachyplesin inhibits tumor growth." Cancer research 61.6 (2001): 2434-2438.
- ↑ 3.0 3.1 Saravanan R, Mohanram H, Joshi M, Domadia PN, Torres J, Ruedl C, Bhattacharjya S. Structure, activity and interactions of the cysteine deleted analog of tachyplesin-1 with lipopolysaccharide micelle: Mechanistic insights into outer-membrane permeabilization and endotoxin neutralization. Biochim Biophys Acta. 2012 Mar 23;1818(7):1613-1624. PMID:22464970 doi:10.1016/j.bbamem.2012.03.015
- ↑ Nakamura, Takanori, et al. "Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure." Journal of Biological Chemistry 263.32 (1988): 16709-16713
- ↑ 5.0 5.1 Kushibiki T, Kamiya M, Aizawa T, Kumaki Y, Kikukawa T, Mizuguchi M, Demura M, Kawabata SI, Kawano K. Interaction between tachyplesin I, an antimicrobial peptide derived from horseshoe crab, and lipopolysaccharide. Biochim Biophys Acta. 2014 Jan 2;1844(3):527-534. doi:, 10.1016/j.bbapap.2013.12.017. PMID:24389234 doi:http://dx.doi.org/10.1016/j.bbapap.2013.12.017
- ↑ 6.0 6.1 6.2 Hong, Jun, et al. "Mechanism of Tachyplesin I injury to bacterial membranes and intracellular enzymes, determined by laser confocal scanning microscopy and flow cytometry." Microbiological research (2014)
- ↑ Yonezawa A, Kuwahara J, Fujii N, Sugiura Y. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry. 1992 Mar 24;31(11):2998-3004. PMID:1372516
- ↑ Lipsky A, Cohen A, Ion A, Yedidia I. Genetic transformation of Ornithogalum via particle bombardment and generation of Pectobacterium carotovorum-resistant plants. Plant Sci. 2014 Nov;228:150-8. doi: 10.1016/j.plantsci.2014.02.002. Epub 2014 Feb, 12. PMID:25438795 doi:http://dx.doi.org/10.1016/j.plantsci.2014.02.002
Proteopedia Page Contributors and Editors (what is this?)
Shulamit Idzikowski, Janak Raj Joshi, Michal Harel, Alexander Berchansky, Joel L. Sussman, Angel Herraez, Jaime Prilusky