Vitis vinifera Flavonoid 3-O-Glucosyltransferase (Vv3GT)
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Your Heading Here (maybe something like 'Structure')== | ==Your Heading Here (maybe something like 'Structure')== | ||
| - | <StructureSection load=' | + | <StructureSection load='2c1z' size='340' side='right' caption='Caption for this structure' scene=''> |
Vitis vinifera Flavonoid 3-O-Glucosyltransferase (Vv3GT) is involved in the modification of grape anthocyanins and thus could affect their color stability. The color plays a significant role in the in agricultural produce, such as table grapes and wine. | Vitis vinifera Flavonoid 3-O-Glucosyltransferase (Vv3GT) is involved in the modification of grape anthocyanins and thus could affect their color stability. The color plays a significant role in the in agricultural produce, such as table grapes and wine. | ||
| - | == | + | == Introduction == |
| + | |||
| + | Vv3GT belongs to Glycosyltransferases (GTs), a large family of enzymes involved in the transfer of sugar residues from a sugar donor to various substrates. Glycosylation of metabolites in plants is usually catalyzed by glycosyltransferases (GTs) belonging to the GT1 sub-family (as classified by the CAZy database [http://www.cazy.org], which use UDP-activated sugars as the major donor molecule and are thus referred to as UGTs. | ||
== Disease == | == Disease == | ||
| Line 10: | Line 12: | ||
== Structural highlights == | == Structural highlights == | ||
| + | |||
| + | Despite low primary sequence similarity, the secondary and tertiary structures of GTs are highly conserved. <ref>PMID:16482224</ref> | ||
| + | |||
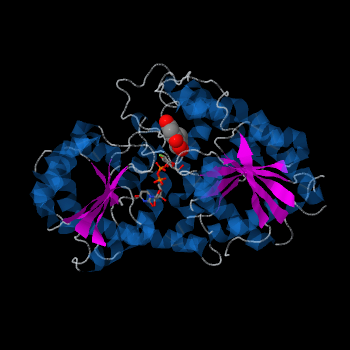
| + | Vv3GT is a GT-B enzyme. GT-B enzymes consists of two β/α/β Rossmann-like domains. The two domains are associated and face each other with the active-site lying between them. These domains are associated with the donor and acceptor substrate binding sites. | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:2C1Z GT-B b.png|frame|alt=Puzzle globe| Vv3GT with the two Rossmann-like domains colored in pink ]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | The plant UGTs are characterized by sharing a highly conserved motif referred to as the PSPG motif (Plant Secondary Product Glycosyltransferase motif). | ||
| + | |||
| + | |||
| + | |||
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
| + | |||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 10:54, 24 January 2015
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Offen W, Martinez-Fleites C, Yang M, Kiat-Lim E, Davis BG, Tarling CA, Ford CM, Bowles DJ, Davies GJ. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 2006 Mar 22;25(6):1396-405. Epub 2006 Feb 16. PMID:16482224