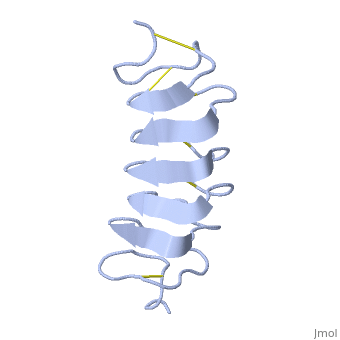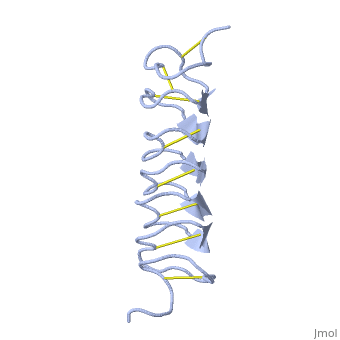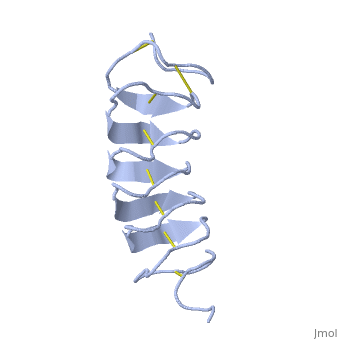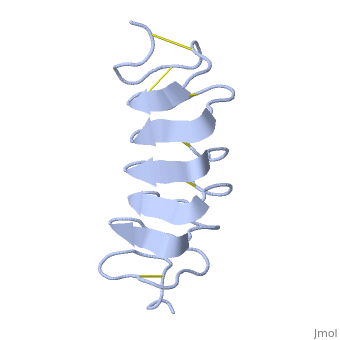
Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
[[Image:406322ad.2.jpg|230px|right]] | [[Image:406322ad.2.jpg|230px|right]] | ||
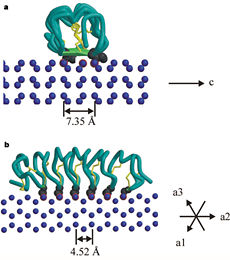
| - | The two dimensional arrays of <scene name='61/612804/Thr/2'> | + | The two dimensional arrays of <scene name='61/612804/Thr/2'>Thr</scene> side chain makes a remarkably good match to the repeated spacing between oxygen atoms in the ice lattice on the prism plane, and a reasonable match to the basal plane, This is reason why the activity of ''Tm''AFP ( Thermal hysteresis) is much higher than the acticity of AFP from Fish (6 celcius degrees and 1 celcius degrees respectively)<ref>doi:10.1016/S0968-0004(01)02028-X</ref> |
'''Lattice matching model for TmAFP binding to ice:''' | '''Lattice matching model for TmAFP binding to ice:''' | ||
| Line 38: | Line 38: | ||
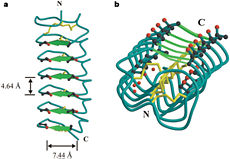
| - | In solution the protein is monomeric and it can bind to ice just in this formation. The protein crystallized as a <scene name='61/612804/Dimer/1'>dimer</scene>, the dimerization occurs along the surface of the beta sheets. The 2 units of the dimer do not directly interact with each other, the contact between them is mediated by highly ordered ranks of water which form hydrogen bonding with Threonine residue. Each water molecule forms two hydrogen bonds to the closer monomer and one to the distant monomer. The distance between two adjacent waters is 4.64±0.20Å the same distance as between Threonine residues 4.64±0.23Å. A good two dimensional match to the ice lattice including all 3 ranks of oxygen atoms (2 from Thr and 1 from water), implying that the ordered water molecules cold act as part of an ice surface and directly participate in the AFP-ice interaction.[[Image:406322ac.2.jpg|300px|right]] The regular array formed by this 3 ranks of oxygen atoms can be seen as a small piece of one layer thick ice to be incorporated into a large ice lattice. There is no need to readjust Threonine side chains, because they don’t present steric interference | + | In solution the protein is monomeric and it can bind to ice just in this formation. The protein crystallized as a <scene name='61/612804/Dimer/1'>dimer</scene>, the dimerization occurs along the surface of the beta sheets. The 2 units of the dimer do not directly interact with each other, the contact between them is mediated by highly ordered ranks of water which form hydrogen bonding with Threonine residue. Each water molecule forms two hydrogen bonds to the closer monomer and one to the distant monomer. The distance between two adjacent waters is 4.64±0.20Å the same distance as between Threonine residues 4.64±0.23Å. A good two dimensional match to the ice lattice including all 3 ranks of oxygen atoms (2 from Thr and 1 from water), implying that the ordered water molecules cold act as part of an ice surface and directly participate in the AFP-ice interaction.[[Image:406322ac.2.jpg|300px|right]] The regular array formed by this 3 ranks of oxygen atoms can be seen as a small piece of one layer thick ice to be incorporated into a large ice lattice. There is no need to readjust Threonine side chains, because they don’t present steric interference <ref name="two">DOI 10.1038/35018604</ref>. The dimer binds threw water molecule which mimice the same way how bindind to ice. This water is very similar in... |
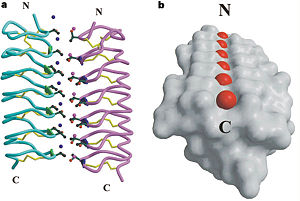
'''Dimer of TmAFP and organization of external water:''' | '''Dimer of TmAFP and organization of external water:''' | ||
| Line 50: | Line 50: | ||
== Function == | == Function == | ||
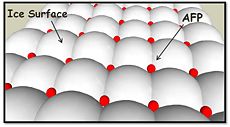
| - | The function of the ''Tm''AFP ( | + | The function of the ''Tm''AFP (and other Antifreeze protein) is TH. TH activity is due to an adsorption inhibition mechanism that’s states that AFPs binds to ice surface and allow ice crystal growths only in surface regions between the bound AFP.[[Image:Unnamed.jpg|230px|right]] Growing curvature causes an increase in surface energy, making the transformation of water into ice lass energetically favorable. Because of that the AFPs lower freezing temperature below the melting point. The difference between the melting and freezing point of the ice called thermal hysteresis activity. |
== The difference between TmAFP (hyperactive AFP) and Type I AFP (moderate AFP) == | == The difference between TmAFP (hyperactive AFP) and Type I AFP (moderate AFP) == | ||
| - | TmAFP has a special structure of beta helix, | + | TmAFP has a special structure of a short very regular beta helix, which yields a binding site consisting of a two dimensional surface: <scene name='61/612804/Thr/2'>two arrays of Thr residues</scene> that can bind to the two planes of ice i.e. prism plane and basal plane. This is why the TH activity can reach 6 Celsius degrees in Hyperactive protein. |
In contrast <scene name='61/612804/Afp1/1'>Type I AFP</scene>, from the fish winter flounder (moderate AFP are usually fish origin), structure is alpha helix allows just one dimensional surface of threonine residue and thus can bind only to one plane of ice (the prism plan). Because of that The TH activity is much lower, maximum TH activity of moderate AFP is 1 Celsius degree. | In contrast <scene name='61/612804/Afp1/1'>Type I AFP</scene>, from the fish winter flounder (moderate AFP are usually fish origin), structure is alpha helix allows just one dimensional surface of threonine residue and thus can bind only to one plane of ice (the prism plan). Because of that The TH activity is much lower, maximum TH activity of moderate AFP is 1 Celsius degree. | ||
Revision as of 15:59, 25 January 2015
| |||||||||||
References
- ↑ Scotter AJ, Marshall CB, Graham LA, Gilbert JA, Garnham CP, Davies PL. The basis for hyperactivity of antifreeze proteins. Cryobiology. 2006 Oct;53(2):229-39. Epub 2006 Aug 2. PMID:16887111 doi:http://dx.doi.org/10.1016/j.cryobiol.2006.06.006
- ↑ Liu K, Jia Z, Chen G, Tung C, Liu R. Systematic size study of an insect antifreeze protein and its interaction with ice. Biophys J. 2005 Feb;88(2):953-8. PMID:15713600 doi:http://dx.doi.org/10.1529/biophysj.104.051169
- ↑ 3.0 3.1 Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ doi: https://dx.doi.org/10.1016/S0968-0004(01)02028-X