We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
RiAFP
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
== Molecular Basis for Ice Binding == | == Molecular Basis for Ice Binding == | ||
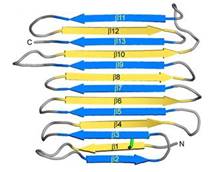
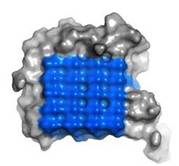
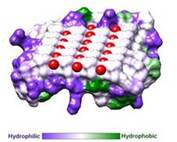
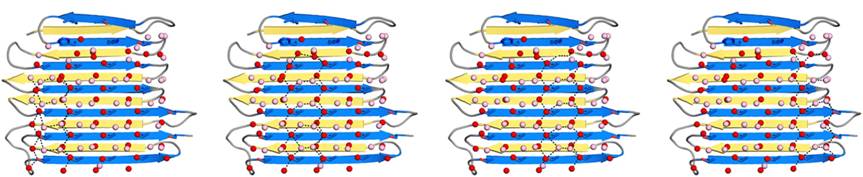
IBS is less hydrophilic than the other β–sheet, which is consistent with its role of interacting with the ice (Figure 3). Adsorption of the AFP ice-binding surface to ice is facilitated by the flatness of the IBS of the ''Ri''AFP. The Sc (shape complementarity) values between ''Ri''AFP and ice interfaces range from 0.75-0.78, where 1.0 indicates perfect match. For comparison, antigen-antibody complexes usually have their Sc values in the range of 0.64–0.68. | IBS is less hydrophilic than the other β–sheet, which is consistent with its role of interacting with the ice (Figure 3). Adsorption of the AFP ice-binding surface to ice is facilitated by the flatness of the IBS of the ''Ri''AFP. The Sc (shape complementarity) values between ''Ri''AFP and ice interfaces range from 0.75-0.78, where 1.0 indicates perfect match. For comparison, antigen-antibody complexes usually have their Sc values in the range of 0.64–0.68. | ||
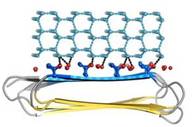
| - | Thr hydroxyls bind 3 ranks of 6 <scene name='60/607864/Isosurface/3'>water molecules</scene> with equivalent spacing between the 4 ranks of Thr side chains. These water molecules are bound tightly, they have lost both translational and rotational freedom and resemble those in an ice lattice. The waters observed in the computational simulation appear to be organized in an ice-like formation, with close matches to the primary prism and basal planes of ice. IBS is responsible for ordering an ice-like array of anchored “clathrate” water molecules to promote adsorption to ice | + | Thr hydroxyls bind 3 ranks of 6 <scene name='60/607864/Isosurface/3'>water molecules</scene> with equivalent spacing between the 4 ranks of Thr side chains. These water molecules are bound tightly, they have lost both translational and rotational freedom and resemble those in an ice lattice. The waters observed in the computational simulation appear to be organized in an ice-like formation, with close matches to the primary prism (Figure 4) and basal planes of ice. IBS is responsible for ordering an ice-like array of anchored “clathrate” water molecules to promote adsorption to ice. |
{|style="margin: 0 auto;" | {|style="margin: 0 auto;" | ||
Revision as of 17:29, 25 January 2015
| |||||||||||
3D structures of antifreeze protein
References
- ↑ Jia Z, Davies PL. Antifreeze proteins: an unusual receptor-ligand interaction. Trends Biochem Sci. 2002 Feb;27(2):101-6. PMID:11852248
- ↑ Chantelle J. Capicciotti, Malay Doshi and Robert N. Ben (2013). Ice Recrystallization Inhibitors: From Biological Antifreezes to Small Molecules, Recent Developments in the Study of Recrystallization, Prof. Peter Wilson (Ed.), ISBN: 978-953-51-0962-4, InTech doi:http://dx.doi.org/10.5772/54992
- ↑ Drori R, Celik Y, Davies PL, Braslavsky I. Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. J R Soc Interface. 2014 Sep 6;11(98):20140526. doi: 10.1098/rsif.2014.0526. PMID:25008081 doi:http://dx.doi.org/10.1098/rsif.2014.0526
- ↑ Raymond JA, DeVries AL. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2589-93. PMID:267952
- ↑ Hakim A, Nguyen JB, Basu K, Zhu DF, Thakral D, Davies PL, Isaacs FJ, Modis Y, Meng W. Crystal structure of an insect antifreeze protein and its implications for ice binding. J Biol Chem. 2013 Apr 26;288(17):12295-304. doi: 10.1074/jbc.M113.450973. Epub, 2013 Mar 12. PMID:23486477 doi:10.1074/jbc.M113.450973