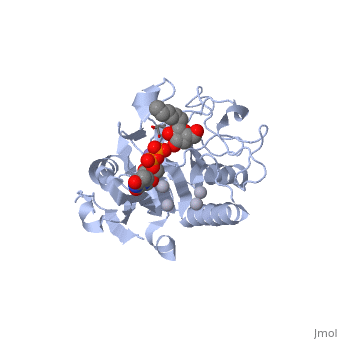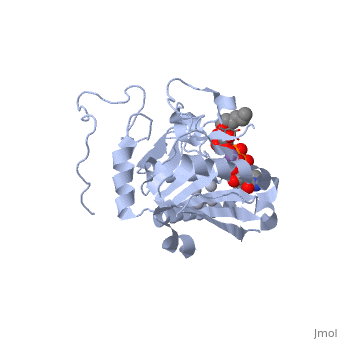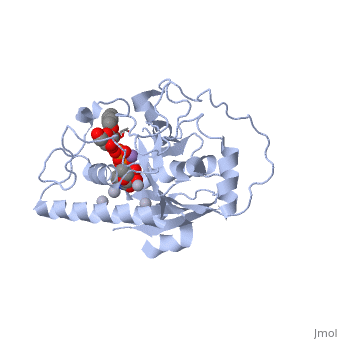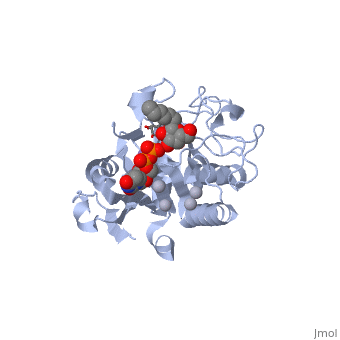
Human ABO(H) blood group glycosyltransferases GTA and GTB catalyze the final monosaccharide addition in the biosynthesis of the human A and B blood group antigens.
Introduction
We can categorize our blood types into 4 groups: A, B, AB, & O, on the surface of red blood cells, there are 2 types of antigen: A & B (each type has its own properties) If a blood cell is type A, the surface of the cell contains Antigens for type A and the body will produce antibodies for type B and vice versa for type B. Type AB contains both antigens on the surface and has neither antibodies. Blood type O has no antigens and thus have both A and B antibodies in its system.
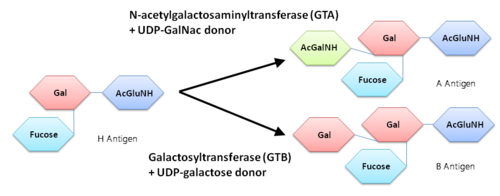
The A and the B antigens were found to be modified from the carbohydrate corresponding to the O blood group by the addition of different monosaccharides, the A antigen was terminated by the sugar N-acetylgalactosmine (GalNAc) while the B antigen was terminated by galactose.
The human ABO(H) blood group antigens are produced by specific glucosyltransferase enzymes (GTs), the ABO blood group system is determined by what type of glucosyltransferases are expressed in the body: N-acetylgalactosaminyltransferase or Galactosyltransferase .
Reaction
An N-acetylgalactosaminyltrasferase (GTA) uses a UDP-GalNac donor to convert the H-antigen acceptor to the A antigen, whereas a galactosyltransferase (GTB) uses UDP-galactose donor to convert the H-antigen acceptor to the B antigen.
Genetics
The ABO gene locus expressing the glycosyltransferases has three main alleleic forms: A, B, and O, the A allele encodes GTA. The B allele encodes GTB, the O allele differs slightly from the A allele by deletion of a single nucleotide. The deletion causes a frameshift and results in translation of an almost entirely different protein that lacks enzymatic activity. This results in H antigen remaining unchanged in case of O groups.
Structural highlights
General topology of GTA and GTB
Both GTs consist of 354 residues and they only differ by four "critical" amino acids.
the polypeptide chain is organized in two domains separated by a cleft, approximately 13 Å wide, containing the active site which consist all four critical amino acid residues. The N-terminal, includes a Rossmann fold and recognizes the nucleotide donor, whereas the disaccharide acceptor binding site is formed by residues in the C-terminal domain in combination with bound UDP.
In the middle of the cleft located a , highly conserved in a large number of glycosyltransferases, which coordinates the Mn2+ ion and was suggested to have a role in catalysis.
H-antigen and UDP substrate binding
Studies show that binding of the donor must precede acceptor binding. Indeed, the donor is a key preliminary step in the formation of the acceptor-binding site as both sugar residues of the acceptor make strong hydrogen bonds with the beta-phosphate group of the UDP.
The donor binds to the enzyme through Phe 121, Ile 123, Tyr 126 and Asp 213.
The H-antigen acceptor interacts with the enzyme primarily through the galactose O4-hydroxyl group, making hydrogen bond interactions with the side chains of His 233 and Glu 303, and the Gal O6-hydroxyl group, which hydrogen bonds to Thr 245 and the Fuc O4'-hydroxyl to Asp 326.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
Structural basis for specifity