2pa2
From Proteopedia
(New page: 200px<br /><applet load="2pa2" size="350" color="white" frame="true" align="right" spinBox="true" caption="2pa2, resolution 2.50Å" /> '''Crystal structure of...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:2pa2.jpg|left|200px]] | + | [[Image:2pa2.jpg|left|200px]] |
| - | + | ||
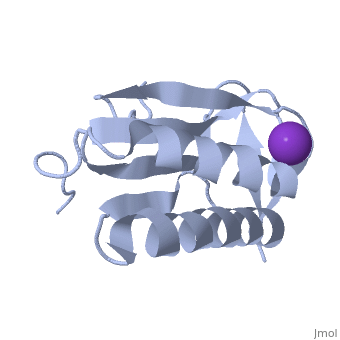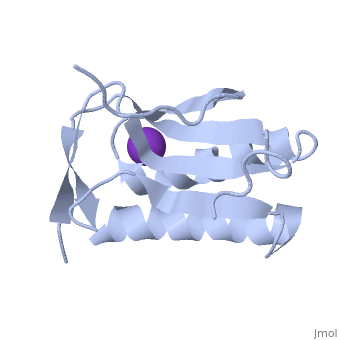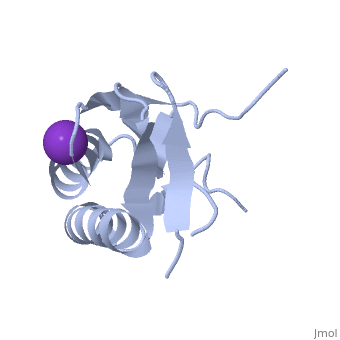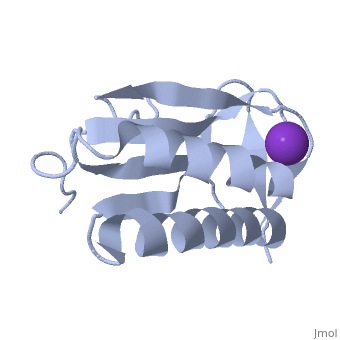
| - | '''Crystal structure of human Ribosomal protein L10 core domain''' | + | {{Structure |
| + | |PDB= 2pa2 |SIZE=350|CAPTION= <scene name='initialview01'>2pa2</scene>, resolution 2.50Å | ||
| + | |SITE= <scene name='pdbsite=AC1:K+Binding+Site+For+Residue+A+1'>AC1</scene> and <scene name='pdbsite=AC2:K+Binding+Site+For+Residue+B+2'>AC2</scene> | ||
| + | |LIGAND= <scene name='pdbligand=K:POTASSIUM ION'>K</scene> | ||
| + | |ACTIVITY= | ||
| + | |GENE= | ||
| + | }} | ||
| + | |||
| + | '''Crystal structure of human Ribosomal protein L10 core domain''' | ||
| + | |||
==Overview== | ==Overview== | ||
| Line 7: | Line 16: | ||
==About this Structure== | ==About this Structure== | ||
| - | 2PA2 is a [ | + | 2PA2 is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2PA2 OCA]. |
==Reference== | ==Reference== | ||
| - | Crystal structure of human ribosomal protein L10 core domain reveals eukaryote-specific motifs in addition to the conserved fold., Nishimura M, Kaminishi T, Takemoto C, Kawazoe M, Yoshida T, Tanaka A, Sugano S, Shirouzu M, Ohkubo T, Yokoyama S, Kobayashi Y, J Mol Biol. 2008 Mar 21;377(2):421-30. Epub 2008 Jan 11. PMID:[http:// | + | Crystal structure of human ribosomal protein L10 core domain reveals eukaryote-specific motifs in addition to the conserved fold., Nishimura M, Kaminishi T, Takemoto C, Kawazoe M, Yoshida T, Tanaka A, Sugano S, Shirouzu M, Ohkubo T, Yokoyama S, Kobayashi Y, J Mol Biol. 2008 Mar 21;377(2):421-30. Epub 2008 Jan 11. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/18258260 18258260] |
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| Line 34: | Line 43: | ||
[[Category: structural genomic]] | [[Category: structural genomic]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 18:10:41 2008'' |
Revision as of 16:10, 20 March 2008
| |||||||
| , resolution 2.50Å | |||||||
|---|---|---|---|---|---|---|---|
| Sites: | and | ||||||
| Ligands: | |||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Crystal structure of human Ribosomal protein L10 core domain
Overview
A phylogenetically conserved ribosomal protein L16p/L10e organizes the architecture of the aminoacyl tRNA binding site on the large ribosomal subunit. Eukaryotic L10 also exhibits a variety of cellular activities, and, in particular, human L10 is known as a putative tumor suppressor, QM. We have determined the 2.5-A crystal structure of the human L10 core domain that corresponds to residues 34-182 of the full-length 214 amino acids. Its two-layered alpha+beta architecture is significantly similar to those of the archaeal and bacterial homologues, substantiating a high degree of structural conservation across the three phylogenetic domains. A cation-binding pocket formed between alpha2 and beta 6 is similar to that of the archaeal L10 protein but appears to be better ordered. Previously reported L10 mutations that cause defects in the yeast ribosome are clustered around this pocket, indicating that its integrity is crucial for its role in L10 function. Characteristic interactions among Arg90-Trp171-Arg139 guide the C-terminal part outside of the central fold, implying that the eukaryote-specific C-terminal extension localizes on the outer side of the ribosome.
About this Structure
2PA2 is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Crystal structure of human ribosomal protein L10 core domain reveals eukaryote-specific motifs in addition to the conserved fold., Nishimura M, Kaminishi T, Takemoto C, Kawazoe M, Yoshida T, Tanaka A, Sugano S, Shirouzu M, Ohkubo T, Yokoyama S, Kobayashi Y, J Mol Biol. 2008 Mar 21;377(2):421-30. Epub 2008 Jan 11. PMID:18258260
Page seeded by OCA on Thu Mar 20 18:10:41 2008
Categories: Homo sapiens | Single protein | Kaminishi, T. | Kawazoe, M. | Kobayashi, Y. | Nishimura, M. | Ohkubo, T. | RSGI, RIKEN Structural Genomics/Proteomics Initiative. | Shirouzu, M. | Sugano, S. | Takemoto, C. | Tanaka, A. | Yokoyama, S. | Yoshida, T. | K | National project on protein structural and functional analyse | Nppsfa | Qm protein | Ribosomal protein l10 | Riken structural genomics/proteomics initiative | Rsgi | Structural genomic