Odorant binding protein
From Proteopedia
| Line 1: | Line 1: | ||
| - | ==Introduction== | ||
| - | Odorant-binding protein (OBP) are soluble proteins which involve in the processes of odorant detection in the olfactory sensilla <ref name="Pelosi 2014">doi: 10.3389/fphys.2014.00320</ref> | ||
| - | |||
| - | Though functionally same, vertebrates and insects OBP have different origin and structure. | ||
| - | OBPs are important for insect olfaction. For instance, OBP76a (LUSH) in the fly [http://en.wikipedia.org/wiki/Drosophila_melanogaster ''Drosophila melanogaster''] is required for the detection of the pheromone vaccenyl acetate <ref name="Xu 2005">doi: 10.1016/j.neuron.2004.12.031</ref> and has been proven to adopt a conformation that activates the odorant receptor <ref name="Laughlin 2008">doi: 10.1016/j.cell.2008.04.046</ref>. | ||
[[Image:Bombyx mori.jpg|thumb|upright=2|''Bombyx mori'', the silk moth, picture by [https://www.flickr.com/photos/depredator007/2522038240/ Fernando Cuenca]]] | [[Image:Bombyx mori.jpg|thumb|upright=2|''Bombyx mori'', the silk moth, picture by [https://www.flickr.com/photos/depredator007/2522038240/ Fernando Cuenca]]] | ||
[[Image:Bombykol.png|thumb|upright=1|Bombykol, a sex pheromone of ''Bombyx mori'', from [http://pubchem.ncbi.nlm.nih.gov/compound/Bombykol#section=Top PubChem]]] | [[Image:Bombykol.png|thumb|upright=1|Bombykol, a sex pheromone of ''Bombyx mori'', from [http://pubchem.ncbi.nlm.nih.gov/compound/Bombykol#section=Top PubChem]]] | ||
| + | ==Introduction== | ||
| + | Odorant-binding protein (OBP) are soluble proteins which involve in the processes of odorant detection in the olfactory sensilla <ref name="Pelosi 2014">doi: 10.3389/fphys.2014.00320</ref>. Though functionally same, vertebrates and insects OBP have different origin and structure. | ||
| + | OBPs are important for insect olfaction. For instance, OBP76a (LUSH) in the fly [http://en.wikipedia.org/wiki/Drosophila_melanogaster ''Drosophila melanogaster''] is required for the detection of the pheromone vaccenyl acetate <ref name="Xu 2005">doi: 10.1016/j.neuron.2004.12.031</ref> and has been proven to adopt a conformation that activates the odorant receptor <ref name="Laughlin 2008">doi: 10.1016/j.cell.2008.04.046</ref>. | ||
==OBP in insects== | ==OBP in insects== | ||
Revision as of 14:07, 28 January 2015

Contents |
Introduction
Odorant-binding protein (OBP) are soluble proteins which involve in the processes of odorant detection in the olfactory sensilla [1]. Though functionally same, vertebrates and insects OBP have different origin and structure. OBPs are important for insect olfaction. For instance, OBP76a (LUSH) in the fly Drosophila melanogaster is required for the detection of the pheromone vaccenyl acetate [2] and has been proven to adopt a conformation that activates the odorant receptor [3].
OBP in insects
OBP Function
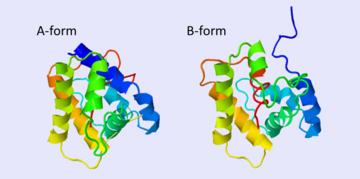
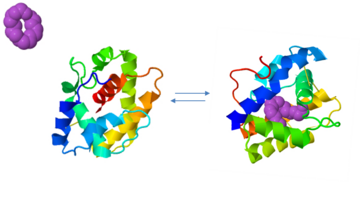
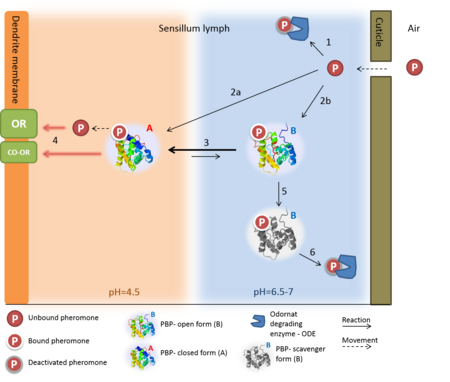
Despite five decades of intensive research, the exact roles of OBP and the mechanism by which the odorant receptor (OR) is activated are still in dispute [4][5].
A few functions have been suggested for OBP:
1. Solubelizing the odorant molecule and its transportation in the sensillar lymph.
2. Protecting the odorant molecule from the odorant degrading enzymes, in the sensillar lymph.
3. Activating of the odorant receptor on the dendrite membrane, by the odorant-OBP complex.
4. Mediating the deactivation of the odorant molecule after the activation of the receptor.
5. An organic anion (the protein has 9 negative charges).
Of all, the first role of OBP as an odorant solubilizer and carrier is generally accepted.
In order to explain the structure and function of these fascinating proteins, this page will further focus on a particular OBP - the well investigated Bombyx mori pheromone binding protein: BmorPBP.
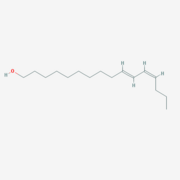
Bombyx mori BmorPBP (lets talk about sex..)
| |||||||||||
See also
- Odorant_binding_protein_3D_structures
- Chemical communication in arthropods
- Pheromone binding protein
References
Proteopedia Page Contributors and Editors (what is this?)
Nurit Eliash, Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky