We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Shai Biran/GFP test page
From Proteopedia
(Difference between revisions)
(New page: == Example page for Green fluorescent protein ("GFP") == <StructureSection load='1ema' size='300' frame='true' side='right' caption='GFP (1ema)' scene=''> [[Image:1ema.gif|thumb|left|...) |
m |
||
| Line 5: | Line 5: | ||
Green fluorescent protein (GFP), originally isolated from the jellyfish Aequorea victoria (PDB entry 1ema), fluorsceses green (509nm) when exposed to blue light (395nm and 475nm). It is one of the most important proteins used in biological research because it can be used to tag otherwise invisible gene products of interest and thus observe their existence, location and movement. | Green fluorescent protein (GFP), originally isolated from the jellyfish Aequorea victoria (PDB entry 1ema), fluorsceses green (509nm) when exposed to blue light (395nm and 475nm). It is one of the most important proteins used in biological research because it can be used to tag otherwise invisible gene products of interest and thus observe their existence, location and movement. | ||
| - | + | Exploring the Structure | |
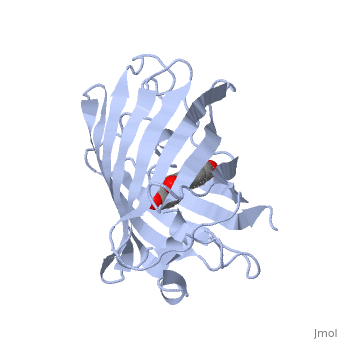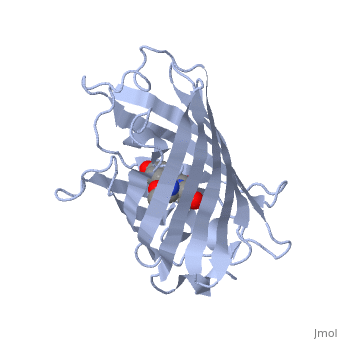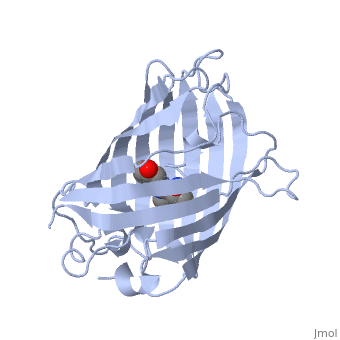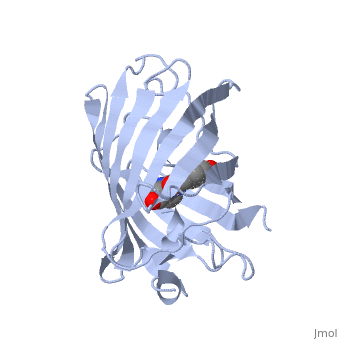
GFP is a beta barrel protein with 11 beta sheets. It is a 26.9kDa protein made up of 238 amino acids. The | GFP is a beta barrel protein with 11 beta sheets. It is a 26.9kDa protein made up of 238 amino acids. The | ||
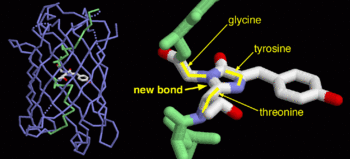
<scene name='Sandbox_4/Green_fp/1'>chromophore</scene>, responsible for the fluorescent properties of the protein, is buried inside the beta barrel as part of the central alpha helix passing through the barrel. The chromophore forms via spontaneous cyclization and oxidation of three residues in the central alpha helix: -Thr65 (or Ser65)-Tyr66-Gly67. This cyclization and oxidation creates the chromophore's five-membered ring via a new bond between the threonine and the glycine residues.<ref>PMID:8703075</ref> | <scene name='Sandbox_4/Green_fp/1'>chromophore</scene>, responsible for the fluorescent properties of the protein, is buried inside the beta barrel as part of the central alpha helix passing through the barrel. The chromophore forms via spontaneous cyclization and oxidation of three residues in the central alpha helix: -Thr65 (or Ser65)-Tyr66-Gly67. This cyclization and oxidation creates the chromophore's five-membered ring via a new bond between the threonine and the glycine residues.<ref>PMID:8703075</ref> | ||
Revision as of 15:29, 28 January 2015
Example page for Green fluorescent protein ("GFP")
| |||||||||||
References
- ↑ Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996 Sep 6;273(5280):1392-5. PMID:8703075