Binding site of AChR
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Basic structure of AChR == | == Basic structure of AChR == | ||
Pentameric ligand gated ion channels (pLGIC), or [http://en.wikipedia.org/wiki/Cys-loop_receptors Cys-loop receptors],are a group of transmembrane ion channel proteins which open to allow ions such as Na+, K+, Ca2+, or Cl- to pass through the membrane in response to the binding of a chemical messenger, such as a neurotransmitter<ref> Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed. Sinauer Associates. pp. 156–7. ISBN 978-0-87893-697-7.</ref>. In overall organization, the <scene name='68/688431/Plgics/1'>pLGICs</scene> have five subunits. The five subunits are arranged in a barrel-like manner around a central symmetry axis that coincides with the ion permeation pathway.<ref>PMID:24167270</ref> In each subunit, the extracellular domin(ECD) of pLGIC encompasses 10β-strands that are organized as a sandwich of two tightly interacting β-sheets, while the transmembrane domain(TMD) folds into a bundle of four α-helices (M1, M2, M3, M4). | Pentameric ligand gated ion channels (pLGIC), or [http://en.wikipedia.org/wiki/Cys-loop_receptors Cys-loop receptors],are a group of transmembrane ion channel proteins which open to allow ions such as Na+, K+, Ca2+, or Cl- to pass through the membrane in response to the binding of a chemical messenger, such as a neurotransmitter<ref> Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed. Sinauer Associates. pp. 156–7. ISBN 978-0-87893-697-7.</ref>. In overall organization, the <scene name='68/688431/Plgics/1'>pLGICs</scene> have five subunits. The five subunits are arranged in a barrel-like manner around a central symmetry axis that coincides with the ion permeation pathway.<ref>PMID:24167270</ref> In each subunit, the extracellular domin(ECD) of pLGIC encompasses 10β-strands that are organized as a sandwich of two tightly interacting β-sheets, while the transmembrane domain(TMD) folds into a bundle of four α-helices (M1, M2, M3, M4). | ||
| + | |||
| + | X-ray structure of homologues of the extracellular domain(ECD) of nAChRs have also been described:the acetylcholine binding protein(AChBP) co-crystallized with agonists and antagonists, and the ECD of α1-nAChRs.<ref>PMID:18987633</ref> | ||
== Superimpose HAP on AChBP == | == Superimpose HAP on AChBP == | ||
| Line 24: | Line 26: | ||
It is noteworthy that the positively charged <scene name='68/688431/Hepes_black/2'>HEPES</scene> molecule shows the location of the acetylcholine binding site and the blockage of passage to this site caused by the toxin. So the ACh binding site in AChBP is assigned by the localization of HEPES. | It is noteworthy that the positively charged <scene name='68/688431/Hepes_black/2'>HEPES</scene> molecule shows the location of the acetylcholine binding site and the blockage of passage to this site caused by the toxin. So the ACh binding site in AChBP is assigned by the localization of HEPES. | ||
| - | + | ||
| Line 32: | Line 34: | ||
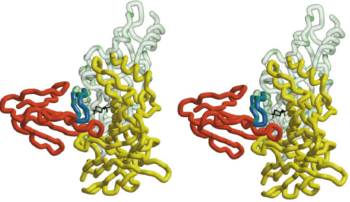
In nAChR, the ligand-binding site is located at the interface between two subunits. The homopentameric α7 receptor contains five identical ligand binding sites. In these sites acrtylcholine is expected to bind through [http://en.wikipedia.org/wiki/Cation%E2%80%93pi_interaction cation-π interactions], where the positive charge of the quaternary ammonium of acetylcholine interacts with the electron-rich aromatic side chains.<ref>PMID:11357122</ref> <scene name='68/688431/Hepes_five_subunits/2'>HEPES</scene> can be refined in the current AChBP structure, it does not make any specific hydrogen bonds with the protein, it stacks with its quaternary ammonium onto <scene name='68/688431/Hepes_trp143/1'>Trp 143</scene> making cation-π interactions as expected for nicotinic agonists.<ref>PMID:11357122</ref> The superimposed model of AChBP and α-BTX suggests that the putative agonist HEPES seen in the AChBP structure is blocked from entering or leaving the AChBP interface cleft by the insertion of <scene name='68/688431/Hepes_black_loop_2/1'>loop 2</scene> of α-BTX into that cleft. This clarifies and explains the strong inhibition of AChR function by the toxin.<ref>PMID:11683996</ref> | In nAChR, the ligand-binding site is located at the interface between two subunits. The homopentameric α7 receptor contains five identical ligand binding sites. In these sites acrtylcholine is expected to bind through [http://en.wikipedia.org/wiki/Cation%E2%80%93pi_interaction cation-π interactions], where the positive charge of the quaternary ammonium of acetylcholine interacts with the electron-rich aromatic side chains.<ref>PMID:11357122</ref> <scene name='68/688431/Hepes_five_subunits/2'>HEPES</scene> can be refined in the current AChBP structure, it does not make any specific hydrogen bonds with the protein, it stacks with its quaternary ammonium onto <scene name='68/688431/Hepes_trp143/1'>Trp 143</scene> making cation-π interactions as expected for nicotinic agonists.<ref>PMID:11357122</ref> The superimposed model of AChBP and α-BTX suggests that the putative agonist HEPES seen in the AChBP structure is blocked from entering or leaving the AChBP interface cleft by the insertion of <scene name='68/688431/Hepes_black_loop_2/1'>loop 2</scene> of α-BTX into that cleft. This clarifies and explains the strong inhibition of AChR function by the toxin.<ref>PMID:11683996</ref> | ||
| - | + | The 13-mer HAP assumes an antiparallel β hairpin structure, and is held snugly between <scene name='68/688431/Fingers_of_btx/1'>fingers 1,2 and 4</scene> of α-BTX. The shortest and most numerous interactions are formed with finger 2 of α-BTX. The intermolecular interaction between finger2 and two arms of the HAP hairpin make the complex stable, like <scene name='68/688431/188arg_and_39val/1'>Arg188 and Val 39</scene>. | |
| - | + | ||
| - | The 13-mer HAP assumes an antiparallel β hairpin structure, and is held snugly between <scene name='68/688431/Fingers_of_btx/1'>fingers 1,2 and 4</scene> of α-BTX. The shortest and most numerous interactions are formed with finger 2 of α-BTX. The intermolecular interaction between finger2 and two arms of the HAP hairpin make the complex stable, like <scene name='68/688431/188arg_and_39val/1'>Arg188 and Val 39</scene>. | + | |
Affinity labeling experiments which indentified position 10 and 33 of -neurotoxin to be with in 11.5-15.5 Å from AChR residues Cys192-Cys193(Michalet et al ., 2000) agree with the α-BTX-HAP structure where the corresponding Cα distance are 11.05 Å(Pro10-Ser193), 12.72 Å(Cys33-Ser193), 14.45 Å(Pro10-Ser 192),9.76 Å(Cys33-Ser192),respectively.<ref>PMID:11683996</ref>So through the complex of α-BTX-HAP, we can see the structure of Acetylcholine binding site. | Affinity labeling experiments which indentified position 10 and 33 of -neurotoxin to be with in 11.5-15.5 Å from AChR residues Cys192-Cys193(Michalet et al ., 2000) agree with the α-BTX-HAP structure where the corresponding Cα distance are 11.05 Å(Pro10-Ser193), 12.72 Å(Cys33-Ser193), 14.45 Å(Pro10-Ser 192),9.76 Å(Cys33-Ser192),respectively.<ref>PMID:11683996</ref>So through the complex of α-BTX-HAP, we can see the structure of Acetylcholine binding site. | ||
Revision as of 08:46, 2 February 2015
| |||||||||||
Quiz
References
- ↑ Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed. Sinauer Associates. pp. 156–7. ISBN 978-0-87893-697-7.
- ↑ Gonzalez-Gutierrez G, Cuello LG, Nair SK, Grosman C. Gating of the proton-gated ion channel from Gloeobacter violaceus at pH 4 as revealed by X-ray crystallography. Proc Natl Acad Sci U S A. 2013 Oct 28. PMID:24167270 doi:http://dx.doi.org/10.1073/pnas.1313156110
- ↑ Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009 Jan 1;457(7225):111-4. Epub 2008 Nov 5. PMID:18987633 doi:10.1038/nature07462
- ↑ Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001 Oct 25;32(2):265-75. PMID:11683996
- ↑ Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001 May 17;411(6835):269-76. PMID:11357122 doi:10.1038/35077011
- ↑ Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001 Oct 25;32(2):265-75. PMID:11683996
- ↑ Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001 May 17;411(6835):269-76. PMID:11357122 doi:10.1038/35077011
- ↑ Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001 May 17;411(6835):269-76. PMID:11357122 doi:10.1038/35077011
- ↑ Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001 Oct 25;32(2):265-75. PMID:11683996
- ↑ Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001 Oct 25;32(2):265-75. PMID:11683996
- ↑ http://en.wikipedia.org/wiki/Nicotinic_acetylcholine_receptor
- ↑ Samson AO, Levitt M. Inhibition mechanism of the acetylcholine receptor by alpha-neurotoxins as revealed by normal-mode dynamics. Biochemistry. 2008 Apr 1;47(13):4065-70. doi: 10.1021/bi702272j. Epub 2008 Mar 8. PMID:18327915 doi:http://dx.doi.org/10.1021/bi702272j
Proteopedia Page Contributors and Editors (what is this?)
Ma Zhuang, Zicheng Ye, Angel Herraez, Alexander Berchansky, Michal Harel