Binding site of AChR
From Proteopedia
(Difference between revisions)
| Line 36: | Line 36: | ||
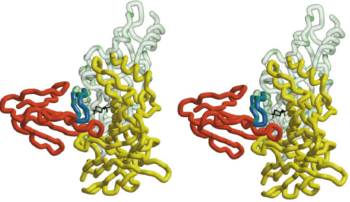
In nAChR, the ligand-binding site is located at the interface between two subunits. The homopentameric α7 receptor contains five identical ligand binding sites. In these sites acrtylcholine is expected to bind through [http://en.wikipedia.org/wiki/Cation%E2%80%93pi_interaction cation-π interactions], where the positive charge of the quaternary ammonium of acetylcholine interacts with the electron-rich aromatic side chains.<ref>PMID:11357122</ref> <scene name='68/688431/Hepes_five_subunits/2'>HEPES</scene> can be refined in the current AChBP structure, it does not make any specific hydrogen bonds with the protein, it stacks with its quaternary ammonium onto <scene name='68/688431/Hepes_trp143/1'>Trp 143</scene> making cation-π interactions as expected for nicotinic agonists.<ref>PMID:11357122</ref> The superimposed model of AChBP and α-BTX suggests that the putative agonist HEPES seen in the AChBP structure is blocked from entering or leaving the AChBP interface cleft by the insertion of <scene name='68/688431/Hepes_black_loop_2/1'>loop 2</scene> of α-BTX into that cleft. This clarifies and explains the strong inhibition of AChR function by the toxin.<ref>PMID:11683996</ref> | In nAChR, the ligand-binding site is located at the interface between two subunits. The homopentameric α7 receptor contains five identical ligand binding sites. In these sites acrtylcholine is expected to bind through [http://en.wikipedia.org/wiki/Cation%E2%80%93pi_interaction cation-π interactions], where the positive charge of the quaternary ammonium of acetylcholine interacts with the electron-rich aromatic side chains.<ref>PMID:11357122</ref> <scene name='68/688431/Hepes_five_subunits/2'>HEPES</scene> can be refined in the current AChBP structure, it does not make any specific hydrogen bonds with the protein, it stacks with its quaternary ammonium onto <scene name='68/688431/Hepes_trp143/1'>Trp 143</scene> making cation-π interactions as expected for nicotinic agonists.<ref>PMID:11357122</ref> The superimposed model of AChBP and α-BTX suggests that the putative agonist HEPES seen in the AChBP structure is blocked from entering or leaving the AChBP interface cleft by the insertion of <scene name='68/688431/Hepes_black_loop_2/1'>loop 2</scene> of α-BTX into that cleft. This clarifies and explains the strong inhibition of AChR function by the toxin.<ref>PMID:11683996</ref> | ||
| - | The 13-mer <scene name='68/688431/Hap/2'>HAP</scene> assumes an antiparallel β hairpin structure. It is held snugly between <scene name='68/688431/Figure_1234/3'>fingers 1,2 and 4</scene> of α-BTX. The shortest and most numerous interactions are formed with <scene name='68/688431/Figure_1234/2'>finger 2</scene> of α-BTX. The two arms of the HAP hairpin assume a β sheet conformation, with residues Leu2 (corresponding to position 188 in AChR)-Tyr4 (corresponding to position 190 in AChR ) making an <scene name='68/688431/Residues_between_btx_and_hap/4'> intermolecular interaction </scene> with α-BTX residues Val39-Glu41 on a loop region. Tyr3 (corresponding to position 189 in AChr) of HAP forms a sung fit into a loop region of α-BTX. The formation of <scene name='68/688431/H_bond_between_hap_and_btx/1'>two H bonds</scene> from its hydroxyl to residues Thr8 and lle11 of α-BTX makes the tyrosine at that position an ideal candidate for forming binding interactions with α-BTX. Indeed, this tyrosine is known to play a crucial role in α-BTX binding. <ref>PMID:11683996</ref> | + | The 13-mer <scene name='68/688431/Hap/2'>HAP</scene> assumes an antiparallel β hairpin structure, which can be used as a model to study the binding site of AChR. It is held snugly between <scene name='68/688431/Figure_1234/3'>fingers 1,2 and 4</scene> of α-BTX. The shortest and most numerous interactions are formed with <scene name='68/688431/Figure_1234/2'>finger 2</scene> of α-BTX. The two arms of the HAP hairpin assume a β sheet conformation, with residues Leu2 (corresponding to position 188 in AChR)-Tyr4 (corresponding to position 190 in AChR ) making an <scene name='68/688431/Residues_between_btx_and_hap/4'> intermolecular interaction </scene> with α-BTX residues Val39-Glu41 on a loop region. Tyr3 (corresponding to position 189 in AChr) of HAP forms a sung fit into a loop region of α-BTX. The formation of <scene name='68/688431/H_bond_between_hap_and_btx/1'>two H bonds</scene> from its hydroxyl to residues Thr8 and lle11 of α-BTX makes the tyrosine at that position an ideal candidate for forming binding interactions with α-BTX. Indeed, this tyrosine is known to play a crucial role in α-BTX binding. <ref>PMID:11683996</ref> |
Revision as of 10:52, 2 February 2015
| |||||||||||
Quiz
References
- ↑ Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed. Sinauer Associates. pp. 156–7. ISBN 978-0-87893-697-7.
- ↑ Gonzalez-Gutierrez G, Cuello LG, Nair SK, Grosman C. Gating of the proton-gated ion channel from Gloeobacter violaceus at pH 4 as revealed by X-ray crystallography. Proc Natl Acad Sci U S A. 2013 Oct 28. PMID:24167270 doi:http://dx.doi.org/10.1073/pnas.1313156110
- ↑ Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009 Jan 1;457(7225):111-4. Epub 2008 Nov 5. PMID:18987633 doi:10.1038/nature07462
- ↑ Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001 Oct 25;32(2):265-75. PMID:11683996
- ↑ Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001 May 17;411(6835):269-76. PMID:11357122 doi:10.1038/35077011
- ↑ Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001 Oct 25;32(2):265-75. PMID:11683996
- ↑ Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001 May 17;411(6835):269-76. PMID:11357122 doi:10.1038/35077011
- ↑ Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001 May 17;411(6835):269-76. PMID:11357122 doi:10.1038/35077011
- ↑ Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001 Oct 25;32(2):265-75. PMID:11683996
- ↑ Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001 Oct 25;32(2):265-75. PMID:11683996
- ↑ http://en.wikipedia.org/wiki/Nicotinic_acetylcholine_receptor
- ↑ Samson AO, Levitt M. Inhibition mechanism of the acetylcholine receptor by alpha-neurotoxins as revealed by normal-mode dynamics. Biochemistry. 2008 Apr 1;47(13):4065-70. doi: 10.1021/bi702272j. Epub 2008 Mar 8. PMID:18327915 doi:http://dx.doi.org/10.1021/bi702272j
Proteopedia Page Contributors and Editors (what is this?)
Ma Zhuang, Zicheng Ye, Angel Herraez, Alexander Berchansky, Michal Harel