We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 992
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
{{Sandbox_gvsu_chm463}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_gvsu_chm463}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
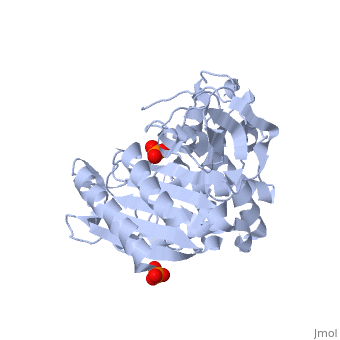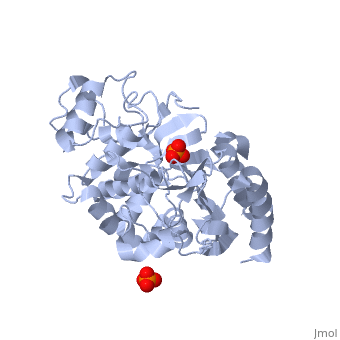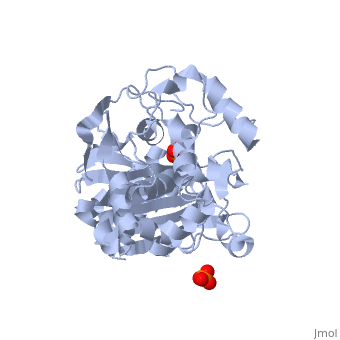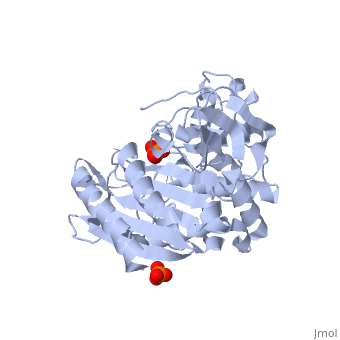
| - | + | Class C β-lactamases are a subcategory of β-lactamase enzymes. These enzymes are produced by some bacteria and result in their resistance to a variety of β-lactam antibiotics. β-lactam antibiotics are classified based on their chemical structure which contains a four membered, amide containing ring known as the β-lactam ring. Class C β-lactamases specifically target cephalosporin antibiotics and deactivate their antimicrobial activity by hydrolyzing the β-lactam ring. | |
<Structure load='1ke4' size='400' frame='true' color='white' align='right' caption='AmpC Class C Beta-lactamase' /> | <Structure load='1ke4' size='400' frame='true' color='white' align='right' caption='AmpC Class C Beta-lactamase' /> | ||
Revision as of 04:15, 25 February 2015
| This Sandbox is Reserved from 20/01/2015, through 30/04/2016 for use in the course "CHM 463" taught by Mary Karpen at the Grand Valley State University. This reservation includes Sandbox Reserved 987 through Sandbox Reserved 996. |
To get started:
More help: Help:Editing |
Class C β-lactamases are a subcategory of β-lactamase enzymes. These enzymes are produced by some bacteria and result in their resistance to a variety of β-lactam antibiotics. β-lactam antibiotics are classified based on their chemical structure which contains a four membered, amide containing ring known as the β-lactam ring. Class C β-lactamases specifically target cephalosporin antibiotics and deactivate their antimicrobial activity by hydrolyzing the β-lactam ring.
|
Background and beta-lactam antibiotics
| |||||||||||