This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 992
From Proteopedia
| Line 20: | Line 20: | ||
== Class C Mechanism == | == Class C Mechanism == | ||
| - | There are four main classes of β-lactamase enzymes, A, B, C, and D. While these main classes all disable the antimicrobial activity of β-lactams by breaking open the β-lactam ring at the amide bond, each class has individually conserved residues that allow the enzyme to maintain catalytic function. Classes A, C and D are most similar by functioning via catalytic serine, while class B functions via catalytic zinc.<ref name = "Bush 2013"> | + | There are four main classes of β-lactamase enzymes, A, B, C, and D. While these main classes all disable the antimicrobial activity of β-lactams by breaking open the β-lactam ring at the amide bond, each class has individually conserved residues that allow the enzyme to maintain catalytic function. Classes A, C and D are most similar by functioning via catalytic serine, while class B functions via catalytic zinc.<ref name="Bush 2013"> |
Bush, Karen. The ABCD’s of β-lactamase nomenclature. J Infect chemother. (2013) 19, 549-559. | Bush, Karen. The ABCD’s of β-lactamase nomenclature. J Infect chemother. (2013) 19, 549-559. | ||
</ref> | </ref> | ||
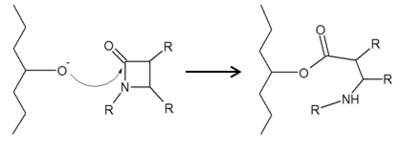
| - | Class C β-lactamases share a very similar mechanism as the Class A β-lactams, acylation followed by hydrolytic deacylation.4 Class C differs from A in that the hydrolytic water, activated by tyrosine 150, approaches the enzyme from the opposite side. This activated water is what allows β-lactamases to deacylation and maintain their catalytic function, while PBPs cannot.<ref name = "Bush 2013" /> | + | Class C β-lactamases share a very similar mechanism as the Class A β-lactams, acylation followed by hydrolytic deacylation.4 Class C differs from A in that the hydrolytic water, activated by tyrosine 150, approaches the enzyme from the opposite side. This activated water is what allows β-lactamases to deacylation and maintain their catalytic function, while PBPs cannot.<ref name="Bush 2013" /> |
[[Image:Beta lactamase mechaism.png|1000px|thumb|center|Class C β-lactamase general mechanism, showing covalently bound β-lactam antibiotic in intermidiate state.]] | [[Image:Beta lactamase mechaism.png|1000px|thumb|center|Class C β-lactamase general mechanism, showing covalently bound β-lactam antibiotic in intermidiate state.]] | ||
Revision as of 05:36, 25 February 2015
| This Sandbox is Reserved from 20/01/2015, through 30/04/2016 for use in the course "CHM 463" taught by Mary Karpen at the Grand Valley State University. This reservation includes Sandbox Reserved 987 through Sandbox Reserved 996. |
To get started:
More help: Help:Editing |
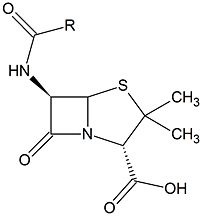
Class C β-lactamases are a subcategory of β-lactamase enzymes. These enzymes are produced by some bacteria and result in their resistance to a variety of β-lactam antibiotics. β-lactam antibiotics are classified based on their chemical structure which contains a four membered, amide containing ring known as the β-lactam ring. Class C β-lactamases specifically target cephalosporin antibiotics and deactivate their antimicrobial activity by hydrolyzing the β-lactam ring.
|
Function and Mechanism
Clinically, β-lactam antibiotics, characterized by their central chemical structure, are utilized to combat bacterial infections by targeting penicillin-binding proteins (PBPs), also known as transpeptidases. PBPs are enzymes that are located in the cell membrane and function in cross-linking to form the peptidoglycan layer. PBPs have a deprotonated serine which executes nucleophilic attack on the carbonyl carbon. The PBP is then covalently attached to one unit of peptidoglycan. The amino group of an alanine on a second unit of peptidoglycan then performs a second nucleophilic attack on the carbonyl carbon, resulting in two covalently cross-linked peptidoglycan units and the regeneration of the catalytic PBP.[1]One of the main causes of resistance to β-lactam drugs is caused by β-lactamases. Chemically, β-lactamases bind to β-lactams the same way β-lactams bind to PBPs. However, the β-lactamases are then able to deactivate the antimicrobial activity of the β-lactams by cleaving the β-lactam bound in the active site through a molecular process called deacylation, rendering it incapable of inhibiting the PBPs and ultimately, allowing cross-linking to occur for adequate cell wall formation.
Class C Mechanism
There are four main classes of β-lactamase enzymes, A, B, C, and D. While these main classes all disable the antimicrobial activity of β-lactams by breaking open the β-lactam ring at the amide bond, each class has individually conserved residues that allow the enzyme to maintain catalytic function. Classes A, C and D are most similar by functioning via catalytic serine, while class B functions via catalytic zinc.[2]
Class C β-lactamases share a very similar mechanism as the Class A β-lactams, acylation followed by hydrolytic deacylation.4 Class C differs from A in that the hydrolytic water, activated by tyrosine 150, approaches the enzyme from the opposite side. This activated water is what allows β-lactamases to deacylation and maintain their catalytic function, while PBPs cannot.[2]
Class C β-lactamases, among many other enzyme types, also contain a structural component known as an oxyanion hole. This pocket of hydrophilic residues directly stabilizes the high-energy tetrahedral intermediate, lowering the activation energy and promoting a faster overall reaction.[3]