This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 992
From Proteopedia
| Line 6: | Line 6: | ||
== Function and Mechanism == | == Function and Mechanism == | ||
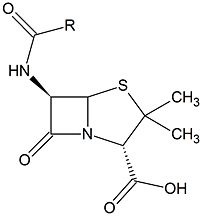
| - | [[Image:Beta-lactam.jpg|200px|thumb|left|A β-lactam antibiotic (Penicillin)]]Clinically, β-lactam antibiotics, characterized by their central chemical structure, are utilized to combat bacterial infections by targeting penicillin-binding proteins (PBPs), also known as transpeptidases. PBPs are enzymes that are located in the cell membrane and function in cross-linking to form the peptidoglycan layer. PBPs have a deprotonated serine which executes nucleophilic attack on the carbonyl carbon. The PBP is then covalently attached to one unit of peptidoglycan. The amino group of an alanine on a second unit of peptidoglycan then performs a second nucleophilic attack on the carbonyl carbon, resulting in two covalently cross-linked peptidoglycan units and the regeneration of the catalytic PBP.<ref>"Peptidoglycan cell wall." The University of Warwick. n.d. Web. 25 Jan 15< | + | [[Image:Beta-lactam.jpg|200px|thumb|left|A β-lactam antibiotic (Penicillin)]]Clinically, β-lactam antibiotics, characterized by their central chemical structure, are utilized to combat bacterial infections by targeting penicillin-binding proteins (PBPs), also known as transpeptidases. PBPs are enzymes that are located in the cell membrane and function in cross-linking to form the peptidoglycan layer. PBPs have a deprotonated serine which executes nucleophilic attack on the carbonyl carbon. The PBP is then covalently attached to one unit of peptidoglycan. The amino group of an alanine on a second unit of peptidoglycan then performs a second nucleophilic attack on the carbonyl carbon, resulting in two covalently cross-linked peptidoglycan units and the regeneration of the catalytic PBP.<ref>"Peptidoglycan cell wall." The University of Warwick. n.d. Web. 25 Jan 15</ref> |
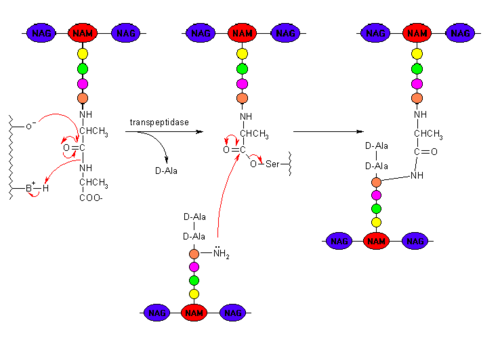
[[Image:Peptidoglycan_cross_linking.png|500px|thumb|right|Peptidoglycan with PDB Cross-linking Mechanism]] | [[Image:Peptidoglycan_cross_linking.png|500px|thumb|right|Peptidoglycan with PDB Cross-linking Mechanism]] | ||
Revision as of 05:38, 25 February 2015
| This Sandbox is Reserved from 20/01/2015, through 30/04/2016 for use in the course "CHM 463" taught by Mary Karpen at the Grand Valley State University. This reservation includes Sandbox Reserved 987 through Sandbox Reserved 996. |
To get started:
More help: Help:Editing |
Class C β-lactamases are a subcategory of β-lactamase enzymes. These enzymes are produced by some bacteria and result in their resistance to a variety of β-lactam antibiotics. β-lactam antibiotics are classified based on their chemical structure which contains a four membered, amide containing ring known as the β-lactam ring. Class C β-lactamases specifically target cephalosporin antibiotics and deactivate their antimicrobial activity by hydrolyzing the β-lactam ring.
|
Function and Mechanism
Clinically, β-lactam antibiotics, characterized by their central chemical structure, are utilized to combat bacterial infections by targeting penicillin-binding proteins (PBPs), also known as transpeptidases. PBPs are enzymes that are located in the cell membrane and function in cross-linking to form the peptidoglycan layer. PBPs have a deprotonated serine which executes nucleophilic attack on the carbonyl carbon. The PBP is then covalently attached to one unit of peptidoglycan. The amino group of an alanine on a second unit of peptidoglycan then performs a second nucleophilic attack on the carbonyl carbon, resulting in two covalently cross-linked peptidoglycan units and the regeneration of the catalytic PBP.[1]The β-lactam ring covalently attaches to PBPs, inhibiting them from executing their role in properly synthesizing the cell wall peptidoglycan layer, via nucleophilic attack of the carbonyl carbon. The β-lactam cannot be removed and thus permanently renders the PBP incapable of its catalytic function in cross-linking. Ultimately, this results in death of bacterial cells from osmotic instability or autolysis.[2]
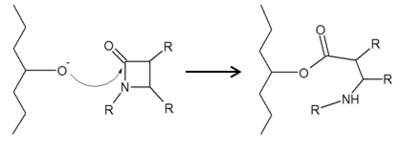
One of the main causes of resistance to β-lactam drugs is caused by β-lactamases. Chemically, β-lactamases bind to β-lactams the same way β-lactams bind to PBPs. However, the β-lactamases are then able to deactivate the antimicrobial activity of the β-lactams by cleaving the β-lactam bound in the active site through a molecular process called deacylation, rendering it incapable of inhibiting the PBPs and ultimately, allowing cross-linking to occur for adequate cell wall formation.
Class C Mechanism
There are four main classes of β-lactamase enzymes, A, B, C, and D. While these main classes all disable the antimicrobial activity of β-lactams by breaking open the β-lactam ring at the amide bond, each class has individually conserved residues that allow the enzyme to maintain catalytic function. Classes A, C and D are most similar by functioning via catalytic serine, while class B functions via catalytic zinc.[3]